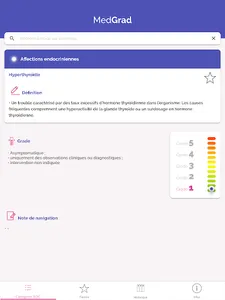
The Common Terminology Criteria for Adverse Events (CTCAE) classification is conducted by the National Cancer Institute. This is a descriptive terminology that can be used for reporting adverse events and with a classification scale (severity) provided for each adverse event term.
MedGrad is an app developed by CHUGAI PHARMA France. An application allowing health professionals easy and fast access to the CTCAE v5.0 classification fully translated into French.
The application allows the search of common terminology criteria for Common Terminology Criteria for Adverse Events categorized.
It allows to visualize in one form all the key information to classify the undesirable events (definition and grades 1 to 5 of severity for each undesirable event)
(For any remark or request for information, we invite users to contact us at marketing@chugai-pharm.fr)
MedGrad is an app developed by CHUGAI PHARMA France. An application allowing health professionals easy and fast access to the CTCAE v5.0 classification fully translated into French.
The application allows the search of common terminology criteria for Common Terminology Criteria for Adverse Events categorized.
It allows to visualize in one form all the key information to classify the undesirable events (definition and grades 1 to 5 of severity for each undesirable event)
(For any remark or request for information, we invite users to contact us at marketing@chugai-pharm.fr)
Show More