myTI
TransPerfect Translations International Inc.
4.6 ★
store rating
Free
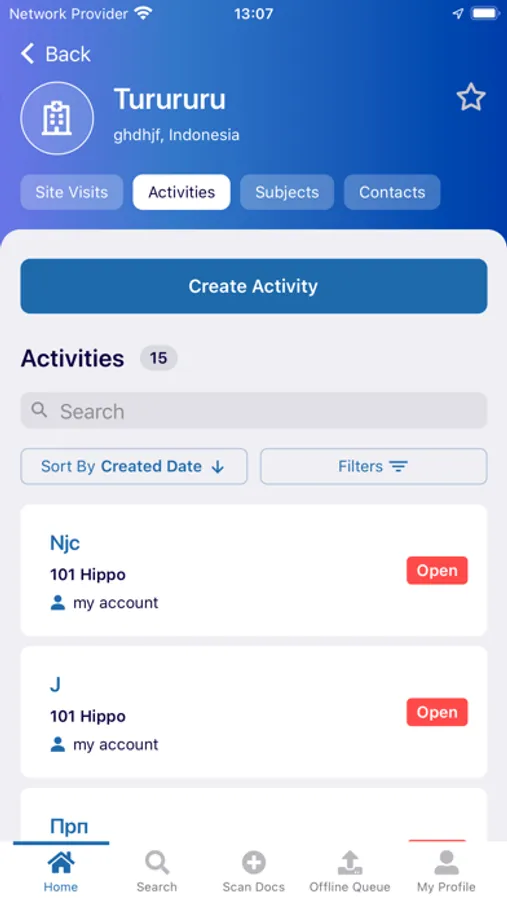
In this clinical trial management app, users can securely capture, upload, and manage documents related to eTMF workflows. Includes biometric login, document status tracking, and query resolution features.
AppRecs review analysis
AppRecs rating 4.6. Trustworthiness 45 out of 100. Review manipulation risk 20 out of 100. Based on a review sample analyzed.
★★★★☆
4.6
AppRecs Rating
Ratings breakdown
5 star
90%
4 star
0%
3 star
0%
2 star
0%
1 star
10%
What to know
⚠
Rating authenticity concerns
High rating concentration (90% 5-star) in sampled ratings
About myTI
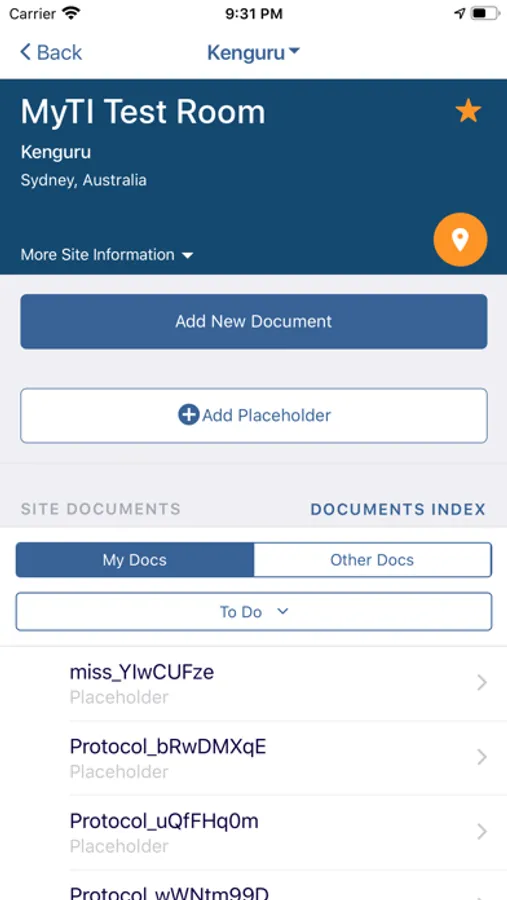
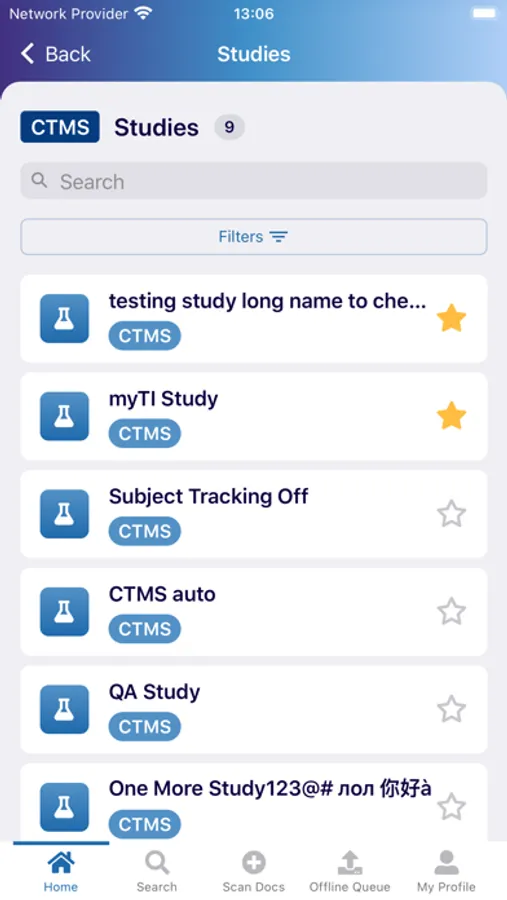
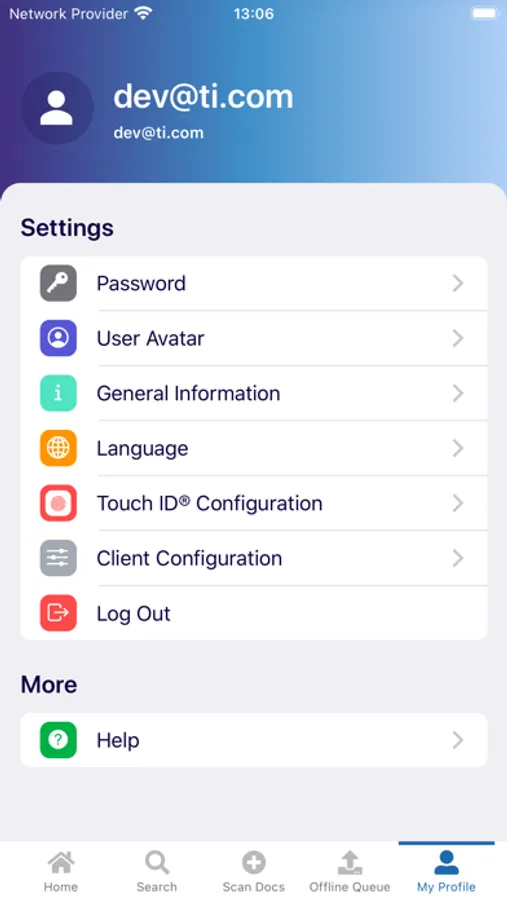
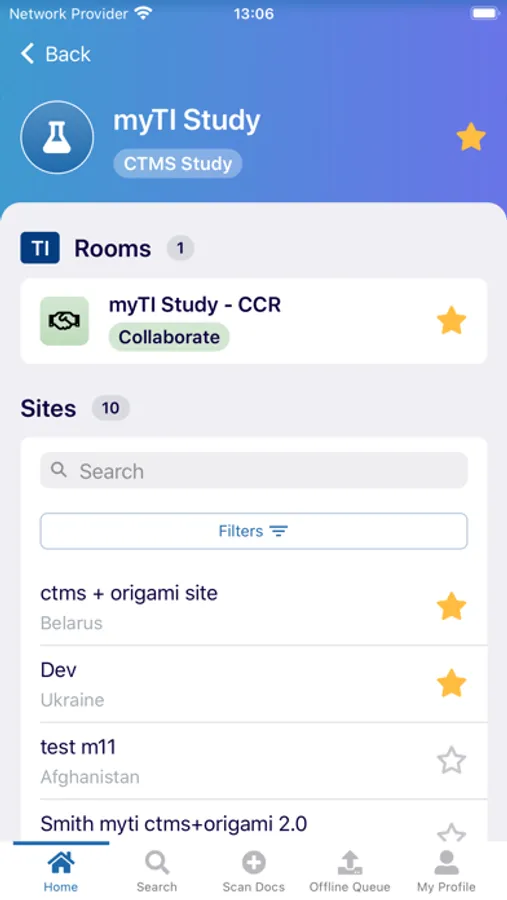
The myTI application is a mobile extension of Trial Interactive, a TransPerfect Life Sciences cloud based e-clinical platform. Trial Interactive was designed by clinical professionals for clinical professionals to meet the end-to-end needs of the clinical trial lifecycle. For the first iteration, myTI enhances the Trial Interactive eTMF module, placing key eTMF processes at the fingertips of study teams at all times. Document capture, query and resolution is essential to a trial’s success and for the health of the eTMF.
Building on the Trial Interactive mission to streamline and simplify global product development, myTI reduces the need to transport large quantities of paper for scanning and expedites the upload of documents. Users login with their biometric login and safely capture documents with their device camera without storing images on the device itself. Captured images are easily uploaded and coded directly to the eTMF. myTI provides constant access to document query and site statuses so users can proactively manage study documentation and data. Users can experience key advantages of eTMF anytime, anywhere they need it. The mobile experience provides role-specific access for all study professionals (CRAs, project managers, TMF leads, etc.).
Highlights of myTI include:
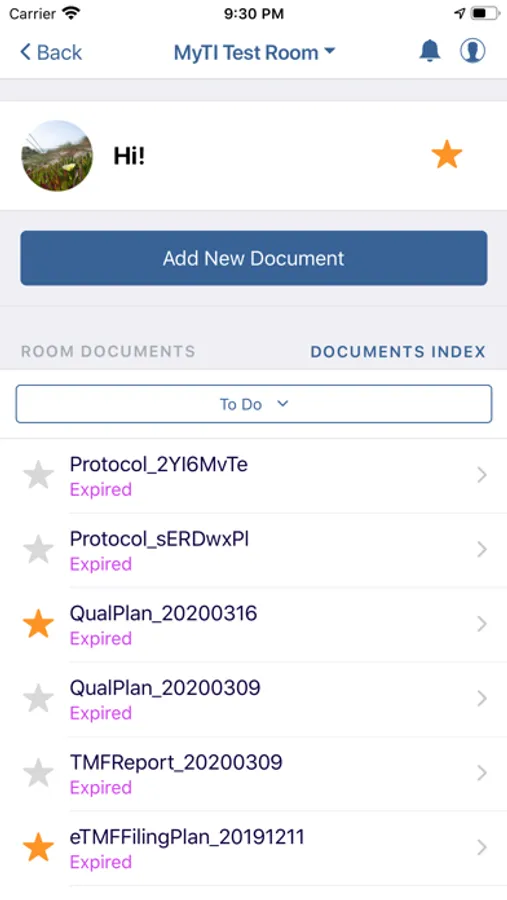
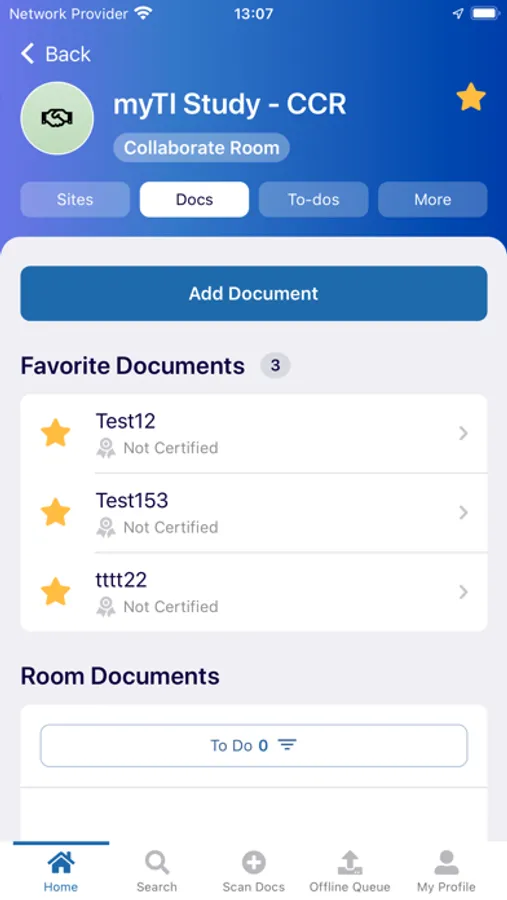
- Document Submission: Securely capture documents with the mobile device camera and add to the eTMF
- Query Resolution: Review documents with queries and resolve them right from the mobile interface
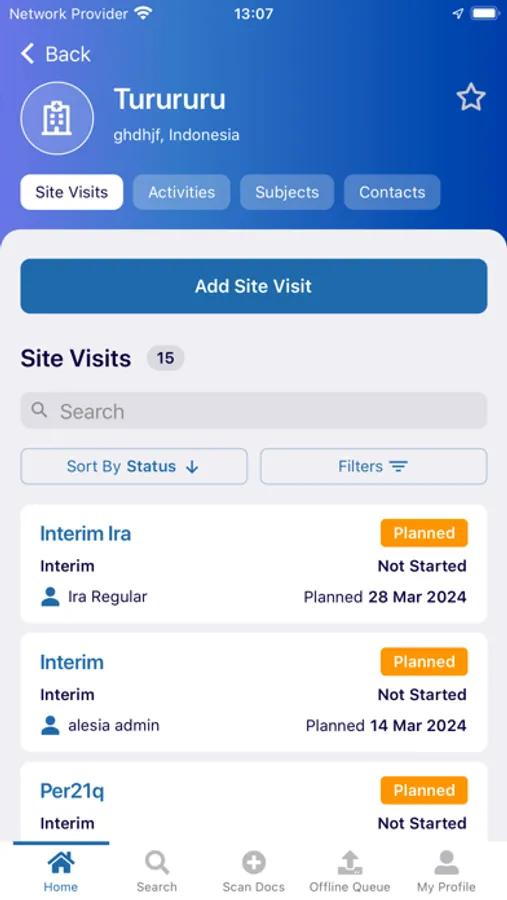
- Document Status: view status, metadata, and update/re-submit expired documents from your phone or tablet
- Cloud-Based: Built on a secure cloud service, myTI sends the images of documents directly into the eTMF
- iPhone Versions 10.3+ with support back to model 5S
- Biometric login: Secure and configurable for devices that support Touch ID® and Face ID®
- Document Detection: Similar to check depositing in banking applications, just hold your mobile device over the document and myTI captures the image immediately, ensuring the image meets quality standards
- Perspective Correction: myTI straightens and adjusts scanned documents prior to converting them into PDF files and uploading into the eTMF
- 21 CFR Part 11 Compliant: Complies with regulatory standards for hosting eTMF documents
About TransPerfect Trial Interactive
TransPerfect’s Trial Interactive solution provides a collaborative web-based platform for study start-up and eTMF that enables sponsors, CROs, IRBs, central laboratories, and other vendors to maintain and update clinical trial documentation in a secure online environment. With fully searchable solutions including investigator portals, Trial Interactive streamlines study timelines and reduces the administrative burdens of global clinical trials. As part of TransPerfect’s Life Sciences division, Trial Interactive is dedicated to working with clients on a global, collaborative level, supporting a wide range of requirements including e-feasibility, eTMF review/reconciliation, pharmacovigilance and safety management, endpoint adjudication, and product licensing and alliance management.
Building on the Trial Interactive mission to streamline and simplify global product development, myTI reduces the need to transport large quantities of paper for scanning and expedites the upload of documents. Users login with their biometric login and safely capture documents with their device camera without storing images on the device itself. Captured images are easily uploaded and coded directly to the eTMF. myTI provides constant access to document query and site statuses so users can proactively manage study documentation and data. Users can experience key advantages of eTMF anytime, anywhere they need it. The mobile experience provides role-specific access for all study professionals (CRAs, project managers, TMF leads, etc.).
Highlights of myTI include:
- Document Submission: Securely capture documents with the mobile device camera and add to the eTMF
- Query Resolution: Review documents with queries and resolve them right from the mobile interface
- Document Status: view status, metadata, and update/re-submit expired documents from your phone or tablet
- Cloud-Based: Built on a secure cloud service, myTI sends the images of documents directly into the eTMF
- iPhone Versions 10.3+ with support back to model 5S
- Biometric login: Secure and configurable for devices that support Touch ID® and Face ID®
- Document Detection: Similar to check depositing in banking applications, just hold your mobile device over the document and myTI captures the image immediately, ensuring the image meets quality standards
- Perspective Correction: myTI straightens and adjusts scanned documents prior to converting them into PDF files and uploading into the eTMF
- 21 CFR Part 11 Compliant: Complies with regulatory standards for hosting eTMF documents
About TransPerfect Trial Interactive
TransPerfect’s Trial Interactive solution provides a collaborative web-based platform for study start-up and eTMF that enables sponsors, CROs, IRBs, central laboratories, and other vendors to maintain and update clinical trial documentation in a secure online environment. With fully searchable solutions including investigator portals, Trial Interactive streamlines study timelines and reduces the administrative burdens of global clinical trials. As part of TransPerfect’s Life Sciences division, Trial Interactive is dedicated to working with clients on a global, collaborative level, supporting a wide range of requirements including e-feasibility, eTMF review/reconciliation, pharmacovigilance and safety management, endpoint adjudication, and product licensing and alliance management.