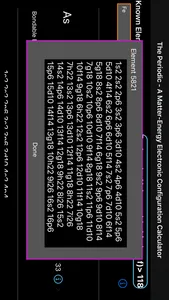
THEPERIODIC , an app that implements the newly discovered and elegant mathematical Universal Quantum Equations (UQEs) that describes the "periodic" and discrete nature of elements. The UQEs mathematically calculates and model the electronic orbital "fingerprint" of all matter known and unknown.... Within these discrete equations that accurately calculates the discrete electronic periodicity of the different types of matter.
Most Periodic Tables published today as it pertains to Electronic Orbital Configuration are in error.
This version of THEPERIODIC adds:
1) The equally elegant Valance Orbital Equation (VOEs) that use the output of the UQEs to calculate the number "normal energy" bonding electrons element will use in reactions with other elements or molecules.
2) The Config Explorer enables examination of elements that have similar outer electronic configuration or valence electron count.
3) The UQEs and VOEs are now neatly implemented in a new Combine enabled framework call ElementalFramework that can models the asynchronous, non-sequential, parallel bonding behavior of biological/chemical reactions.
4) Numerous discrepancies (Periodic Table errors) have been exposed by the mathematical computation from the UQEs. One very important error is with iron (Fe). In most chemistry textbooks is shows Fe with a valence of 2 or 3. The VOEs calculates that Fe "normal energy" bonding electrons are 4! This exactly matches what is seen in the energy powerhouse hemoglobin used by animals.
Also the published structure of Fe2O3 is wrong in most textbooks the textbooks and should look like a 3D polygon
O1
/ .
Fe 1. - Fe2
/
(bond to Fe1) O2 . / (bond to Fe2)
. /
/
O3
is but just one example. UQEs states the valence of Fe is 4 (and verified by nature's Hemoglobin molecule. It is not 2, or 3 as most texts suggests.
The larger elements are more apt to use other unfilled orbital shells to "achieve closer nobility" by capturing smaller element electrons (along with the other element and thus the definition of bond). THEPERIODIC's (VOEs) is a baseline, not rigid guide on how, particularly, the bigger elements may bond.
The ElementalFramework or "SOURCE OF ELEMENTAL TRUTH" exposes its partial strength, in THEPERIODIC,. The UQEs mathematically calculates important elemental information needed by anyone (e.g. students, scientists, engineers, etc...) that are tired of the laborious (now unnecessary) error prone task of figuring out the electronic orbital configuration of elements! THEPERIODIC replaces the honored, but now outdated/with apparent errors legacy Periodic Table.
Let's continue to have more "FUN"/exploring with new "clearer vision" using math-based Universal Quantum Equations (UQEs) and Valance Orbital Equation (VOEs) that mathematically describes all known and unknown elements electronic configurations and valences....
Most Periodic Tables published today as it pertains to Electronic Orbital Configuration are in error.
This version of THEPERIODIC adds:
1) The equally elegant Valance Orbital Equation (VOEs) that use the output of the UQEs to calculate the number "normal energy" bonding electrons element will use in reactions with other elements or molecules.
2) The Config Explorer enables examination of elements that have similar outer electronic configuration or valence electron count.
3) The UQEs and VOEs are now neatly implemented in a new Combine enabled framework call ElementalFramework that can models the asynchronous, non-sequential, parallel bonding behavior of biological/chemical reactions.
4) Numerous discrepancies (Periodic Table errors) have been exposed by the mathematical computation from the UQEs. One very important error is with iron (Fe). In most chemistry textbooks is shows Fe with a valence of 2 or 3. The VOEs calculates that Fe "normal energy" bonding electrons are 4! This exactly matches what is seen in the energy powerhouse hemoglobin used by animals.
Also the published structure of Fe2O3 is wrong in most textbooks the textbooks and should look like a 3D polygon
O1
/ .
Fe 1. - Fe2
/
(bond to Fe1) O2 . / (bond to Fe2)
. /
/
O3
is but just one example. UQEs states the valence of Fe is 4 (and verified by nature's Hemoglobin molecule. It is not 2, or 3 as most texts suggests.
The larger elements are more apt to use other unfilled orbital shells to "achieve closer nobility" by capturing smaller element electrons (along with the other element and thus the definition of bond). THEPERIODIC's (VOEs) is a baseline, not rigid guide on how, particularly, the bigger elements may bond.
The ElementalFramework or "SOURCE OF ELEMENTAL TRUTH" exposes its partial strength, in THEPERIODIC,. The UQEs mathematically calculates important elemental information needed by anyone (e.g. students, scientists, engineers, etc...) that are tired of the laborious (now unnecessary) error prone task of figuring out the electronic orbital configuration of elements! THEPERIODIC replaces the honored, but now outdated/with apparent errors legacy Periodic Table.
Let's continue to have more "FUN"/exploring with new "clearer vision" using math-based Universal Quantum Equations (UQEs) and Valance Orbital Equation (VOEs) that mathematically describes all known and unknown elements electronic configurations and valences....
Show More