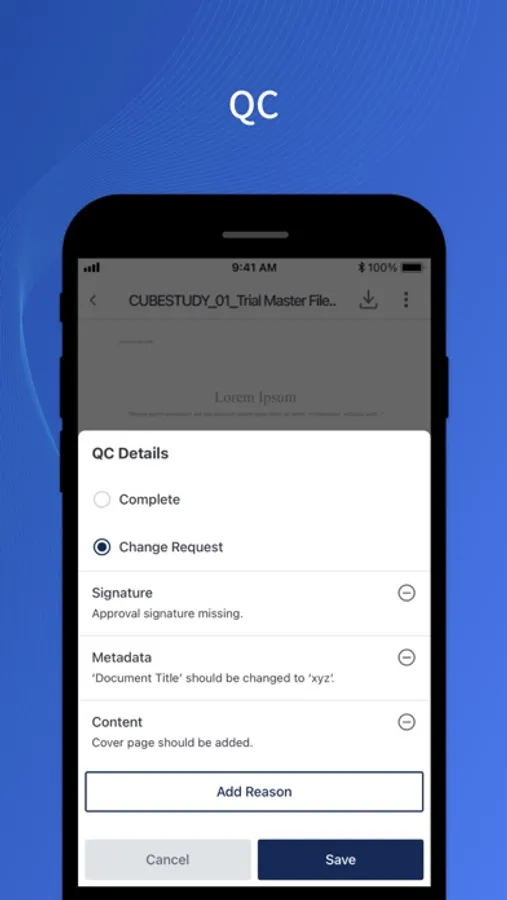
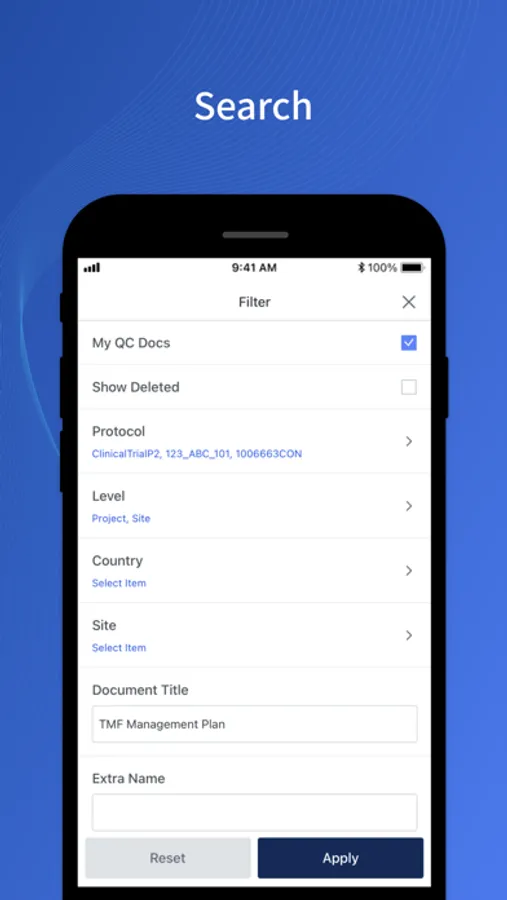
About cubeTMFm
cubeTMFm is a mobile app developed to provide smartphone access to cubeTMF® web services — CRScube’s trial master file (TMF) management solution for clinical trials.
App Features:
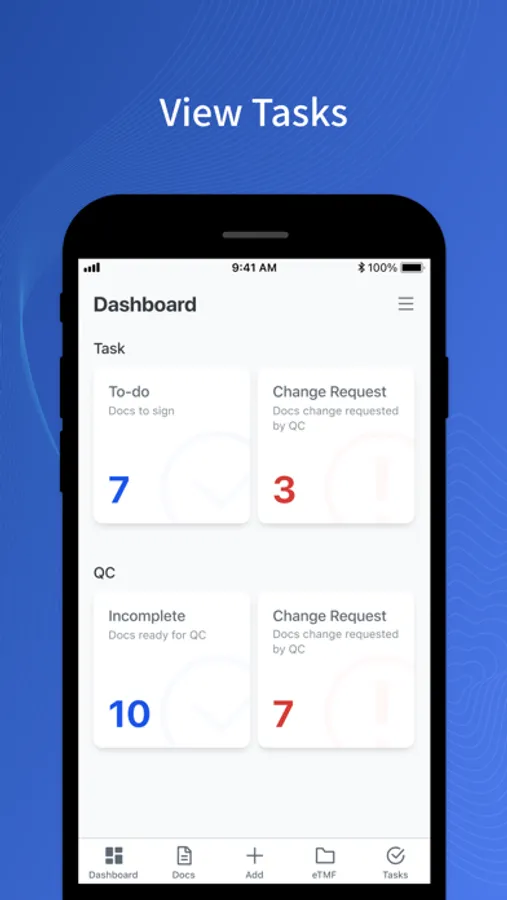
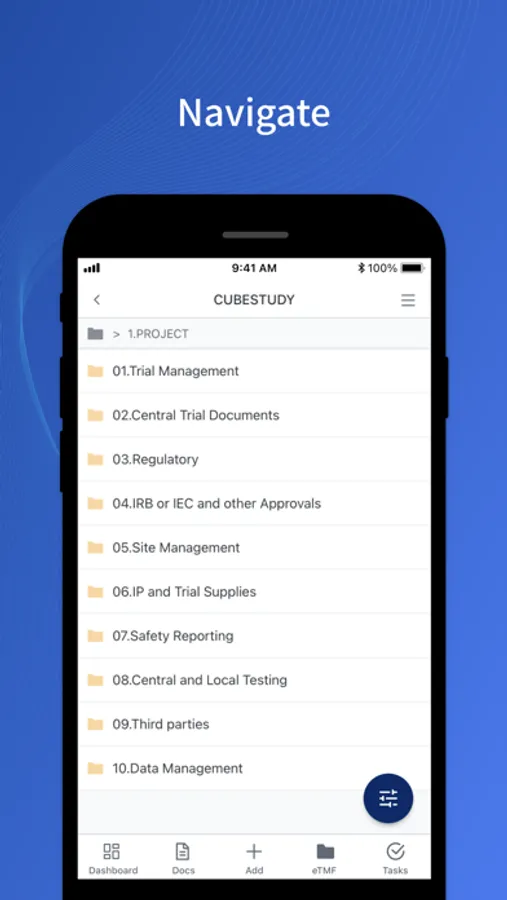
- Access and download TMF documents.
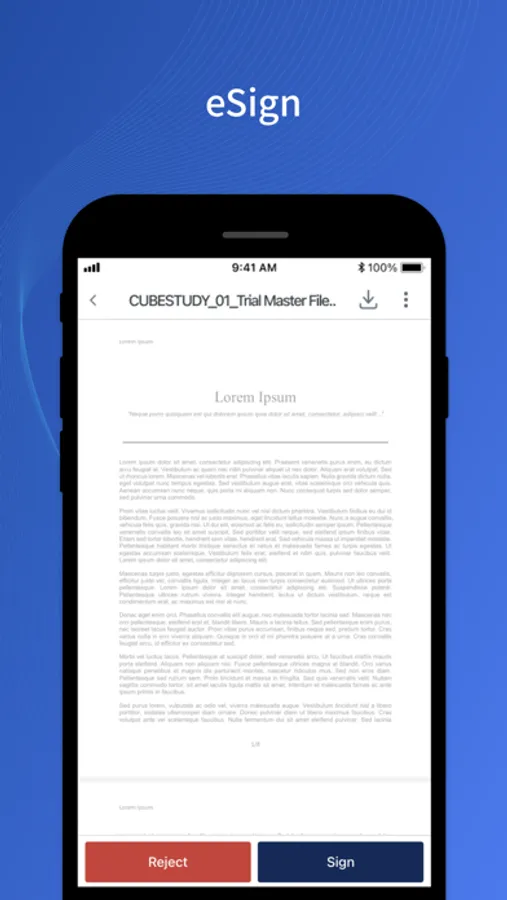
- eSign and QC documents per automated workflows.
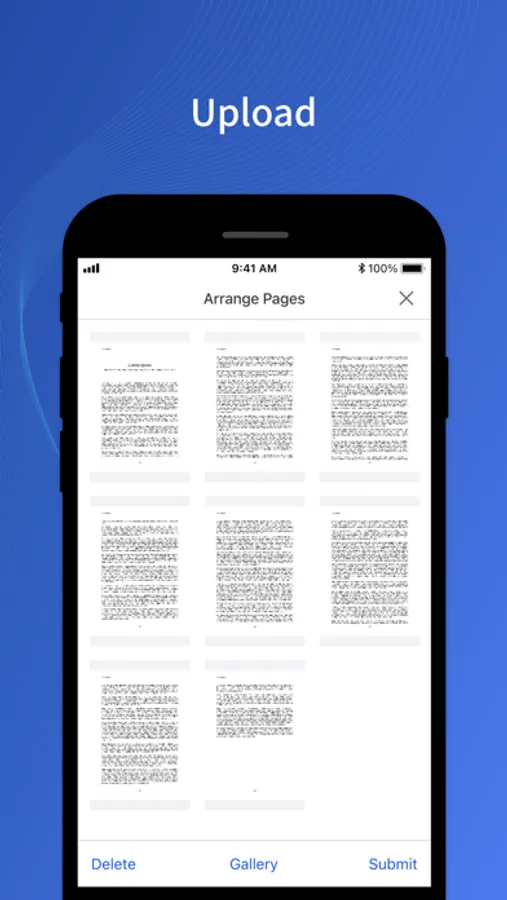
- Upload document files using PDFs or images.
About the Web App:
cubeTMF® is CRScube’s web application for paperless trial master file (TMF) management. cubeTMF is fully scalable and exists securely on the cloud for global centralized access.
The system provides a simple, user-friendly interface and end-to-end features for constructing and managing a TMF repository as well as processing documents through automated eSign and QC workflows.
CRScube:
CRScube provides a comprehensive suite of eClinical solutions to CROs, Biotech, Pharmaceutical, Academic, and Non-Profit organizations globally. We are dedicated to providing the latest eClinical technology that is fast, flexible, and easy-to-use to streamline end-to-end operations for Phase I-IV, medical device, and Investigator-Initiated Trials.
App Features:
- Access and download TMF documents.
- eSign and QC documents per automated workflows.
- Upload document files using PDFs or images.
About the Web App:
cubeTMF® is CRScube’s web application for paperless trial master file (TMF) management. cubeTMF is fully scalable and exists securely on the cloud for global centralized access.
The system provides a simple, user-friendly interface and end-to-end features for constructing and managing a TMF repository as well as processing documents through automated eSign and QC workflows.
CRScube:
CRScube provides a comprehensive suite of eClinical solutions to CROs, Biotech, Pharmaceutical, Academic, and Non-Profit organizations globally. We are dedicated to providing the latest eClinical technology that is fast, flexible, and easy-to-use to streamline end-to-end operations for Phase I-IV, medical device, and Investigator-Initiated Trials.