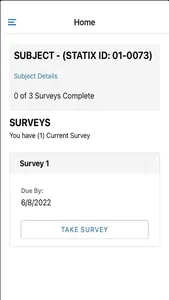
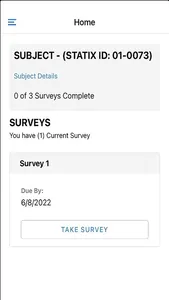
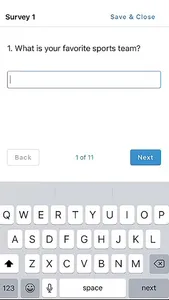
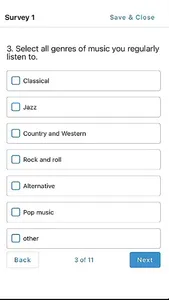
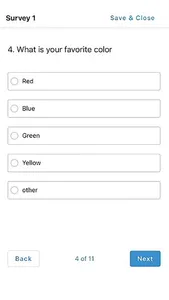
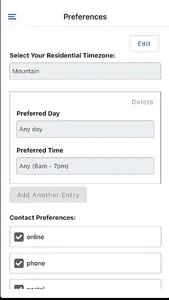
STATIX mobile app is available to participants enrolled in research studies managed by STATIX, LLC. The app facilitates convenient data collection optimized for clinical studies and research registries, including electronic patient-reported outcomes (ePRO.) Key features include seamless integration with our broader data collection service, push notifications to remind participants complete follow-up surveys when requested, editable contact information and preference, and the ability to complete follow-up surveys through the app based on the study survey protocol.
The app meets the FDA's Guidance for Computerized Systems Used in Clinical Investigations, Guidance for Electronic Source Data in Clinical Investigations, and Guidance for Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. It is integrated with STATIX's broader suite of online tools combined with a data collection service to create, manage, and systematically collect longitudinal research data for multi-center clinical studies and research registries.
The app meets the FDA's Guidance for Computerized Systems Used in Clinical Investigations, Guidance for Electronic Source Data in Clinical Investigations, and Guidance for Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. It is integrated with STATIX's broader suite of online tools combined with a data collection service to create, manage, and systematically collect longitudinal research data for multi-center clinical studies and research registries.
Show More