About Nimbus Health
IMPORTANT NOTE: This application is not a medical device and is not intended for use in the diagnosis or treatment of any medical condition and is not regulated by the FDA.
Please seek a doctor’s advice before making any medical decisions and this app is not for making medical decisions.
Nimbus Health's mission is to redefine the standard of care in pulmonary medicine and deliver the best lung care in America. The Nimbus Health technology platform includes this app focused on patients to record data from a Spirometer and provide reminders for recordings. This app is for recording data only and is not a medical device. The Nimbus App uses MIR Spirometers under a licensing agreement between Nimbus Health INC and MIR srl Medical International. The app also connects to an Adherium inhaler with agreement from a Adherium North America, Inc. The app also connects to a Yirdoc nebulizer to obtain usage information with agreement from Yirdoc.
Since the issuance of the guidance document in 2015, section 3060(a) of the 21st Century Cures Act (Cures Act) amended section 520 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) on December 13, 2016, removing certain software functions from the definition of device in section 201(h) of the FD&C Act. Specifically, pursuant to section 520(o)(1)(D) of the FD&C Act, software functions that are solely intended to transfer, store, convert formats, or display medical device data or medical imaging data, unless the software function is intended to interpret or analyze clinical laboratory test or other device data, results, and findings, are not devices and are not subject to FDA laws and regulations applicable to devices.”
This app is intended to transfer device data from an FDA approved device and is not subject to FDA laws and regulations applicable to devices
Features:
- Access to this app is only allowed and created by a Nimbus Health employee during a consultation and Nimbus Health program enrollment.
- Push notifications to reminder the user to take a spirometry recording
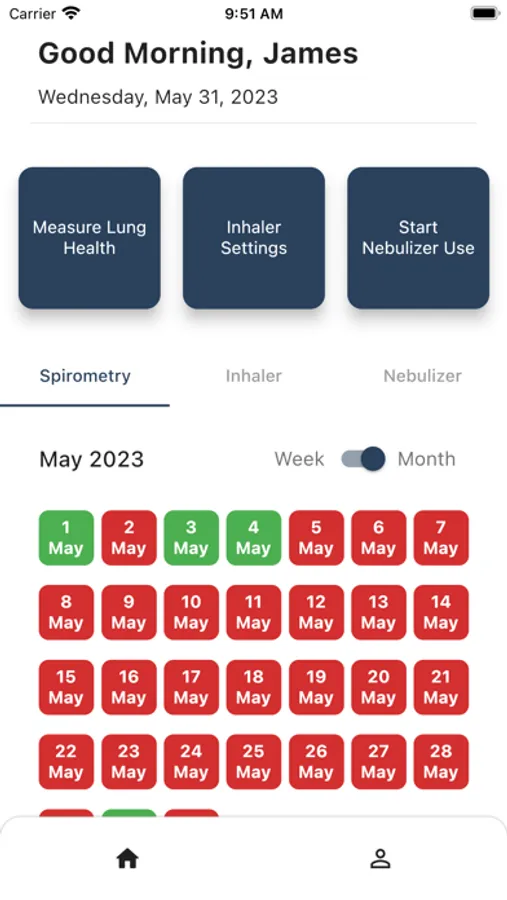
- Historical Calendar view of Spirometer recordings
- App automatically connects to an MIR spirometer with Bluetooth low energy
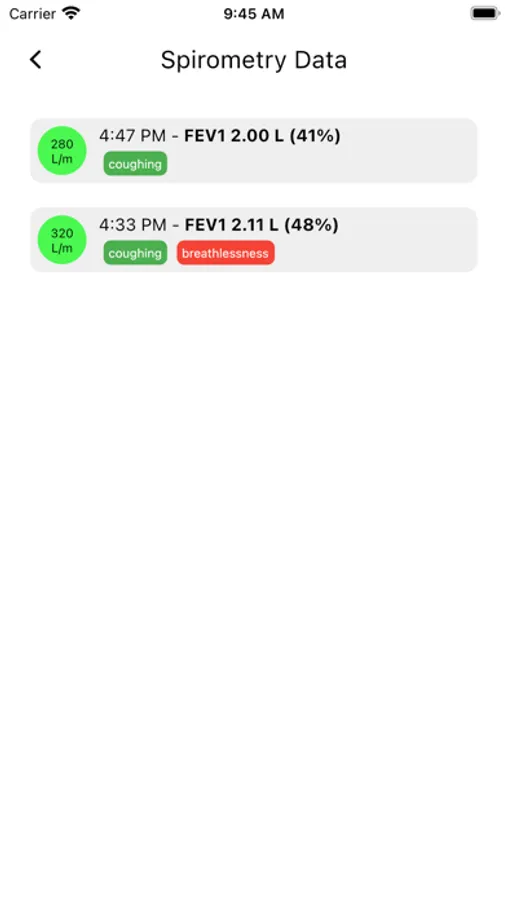
- This app uses an MIR Spirometer device to make Spirometer recordings Spirometry recordings may include: PEF, FVC, FEV1, FEV1/FVC ratio, FEF25/75, FEV6, VEXT, PEFTIME, FEF75, FEF25, FEF50
- App collects user recorded symptoms when a Spirometer is recorded and is displayed to the user
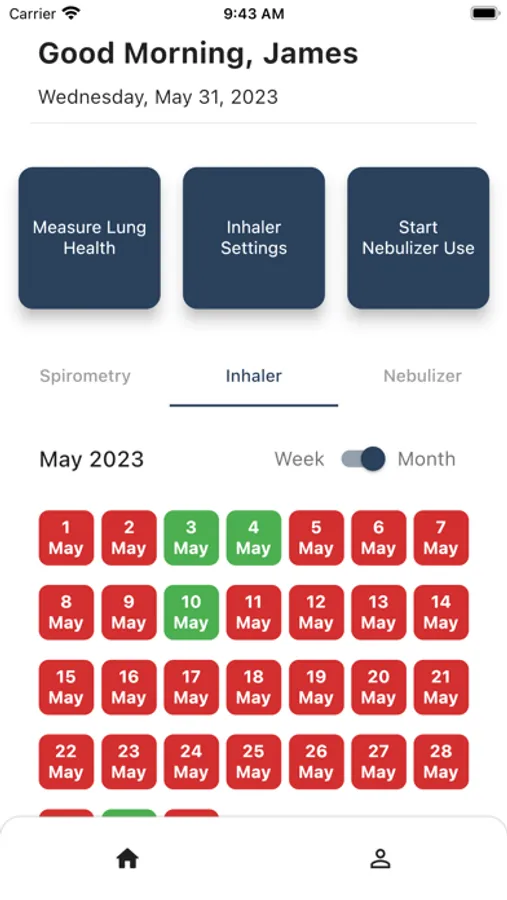
- App collects user recorded usage when an inhaler is recorded and is displayed to the user
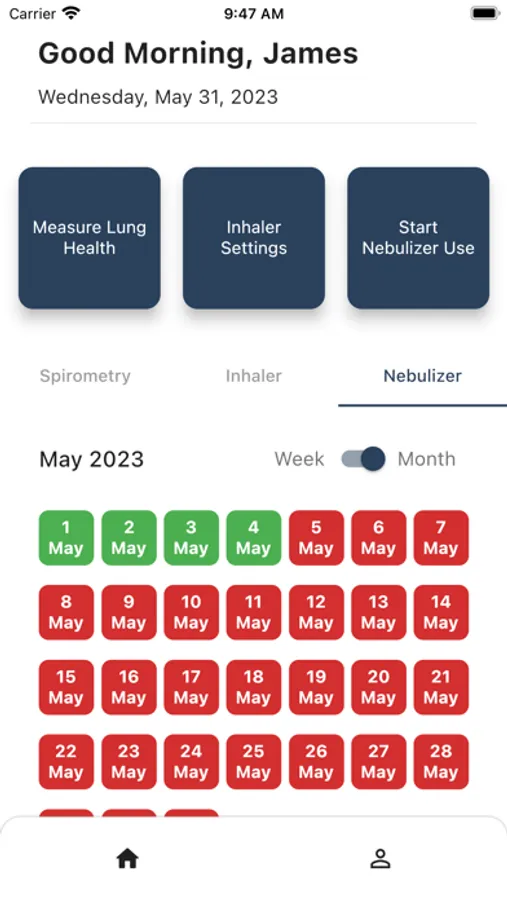
- App collects Spirometer usage information on/off and breaths.
Accuracy:
- The Spirometer are designed and manufactured by MIR srl Medical International Research, a world leader for innovation with more than 25 years experience in spirometry, oximetry and mobile health.
- MIR spirometers are compliant with ATS/ERS guidelines, ISO 23747:2015 (for Peak Flow), ISO 22782:2009 (for Spirometry), ISO 80601-2-61 (for Oximetry).
- The MIR Spirometer has received regulatory clearance for US market (FDA).
- The Adherium inhaler has received regulatory clearance for US market (FDA).
- The Yirdoc nebulizer has received regulatory clearance for US market (FDA).
Personal data:
- Data is saved exclusively in the Nimbus Health HIPAA compliant platform
- Data is not sent to any third party unless you decide to do so.
- Personal data (Name, Date of birth) is displayed by the app with the purpose of calculating target values for spirometry.
Please seek a doctor’s advice before making any medical decisions and this app is not for making medical decisions.
Nimbus Health's mission is to redefine the standard of care in pulmonary medicine and deliver the best lung care in America. The Nimbus Health technology platform includes this app focused on patients to record data from a Spirometer and provide reminders for recordings. This app is for recording data only and is not a medical device. The Nimbus App uses MIR Spirometers under a licensing agreement between Nimbus Health INC and MIR srl Medical International. The app also connects to an Adherium inhaler with agreement from a Adherium North America, Inc. The app also connects to a Yirdoc nebulizer to obtain usage information with agreement from Yirdoc.
Since the issuance of the guidance document in 2015, section 3060(a) of the 21st Century Cures Act (Cures Act) amended section 520 of the Federal Food, Drug, and Cosmetic Act (FD&C Act) on December 13, 2016, removing certain software functions from the definition of device in section 201(h) of the FD&C Act. Specifically, pursuant to section 520(o)(1)(D) of the FD&C Act, software functions that are solely intended to transfer, store, convert formats, or display medical device data or medical imaging data, unless the software function is intended to interpret or analyze clinical laboratory test or other device data, results, and findings, are not devices and are not subject to FDA laws and regulations applicable to devices.”
This app is intended to transfer device data from an FDA approved device and is not subject to FDA laws and regulations applicable to devices
Features:
- Access to this app is only allowed and created by a Nimbus Health employee during a consultation and Nimbus Health program enrollment.
- Push notifications to reminder the user to take a spirometry recording
- Historical Calendar view of Spirometer recordings
- App automatically connects to an MIR spirometer with Bluetooth low energy
- This app uses an MIR Spirometer device to make Spirometer recordings Spirometry recordings may include: PEF, FVC, FEV1, FEV1/FVC ratio, FEF25/75, FEV6, VEXT, PEFTIME, FEF75, FEF25, FEF50
- App collects user recorded symptoms when a Spirometer is recorded and is displayed to the user
- App collects user recorded usage when an inhaler is recorded and is displayed to the user
- App collects Spirometer usage information on/off and breaths.
Accuracy:
- The Spirometer are designed and manufactured by MIR srl Medical International Research, a world leader for innovation with more than 25 years experience in spirometry, oximetry and mobile health.
- MIR spirometers are compliant with ATS/ERS guidelines, ISO 23747:2015 (for Peak Flow), ISO 22782:2009 (for Spirometry), ISO 80601-2-61 (for Oximetry).
- The MIR Spirometer has received regulatory clearance for US market (FDA).
- The Adherium inhaler has received regulatory clearance for US market (FDA).
- The Yirdoc nebulizer has received regulatory clearance for US market (FDA).
Personal data:
- Data is saved exclusively in the Nimbus Health HIPAA compliant platform
- Data is not sent to any third party unless you decide to do so.
- Personal data (Name, Date of birth) is displayed by the app with the purpose of calculating target values for spirometry.