Egg With Vinegar
Sunway-Network Inc
Free
0+
downloads
About Egg With Vinegar
Experimental principle:
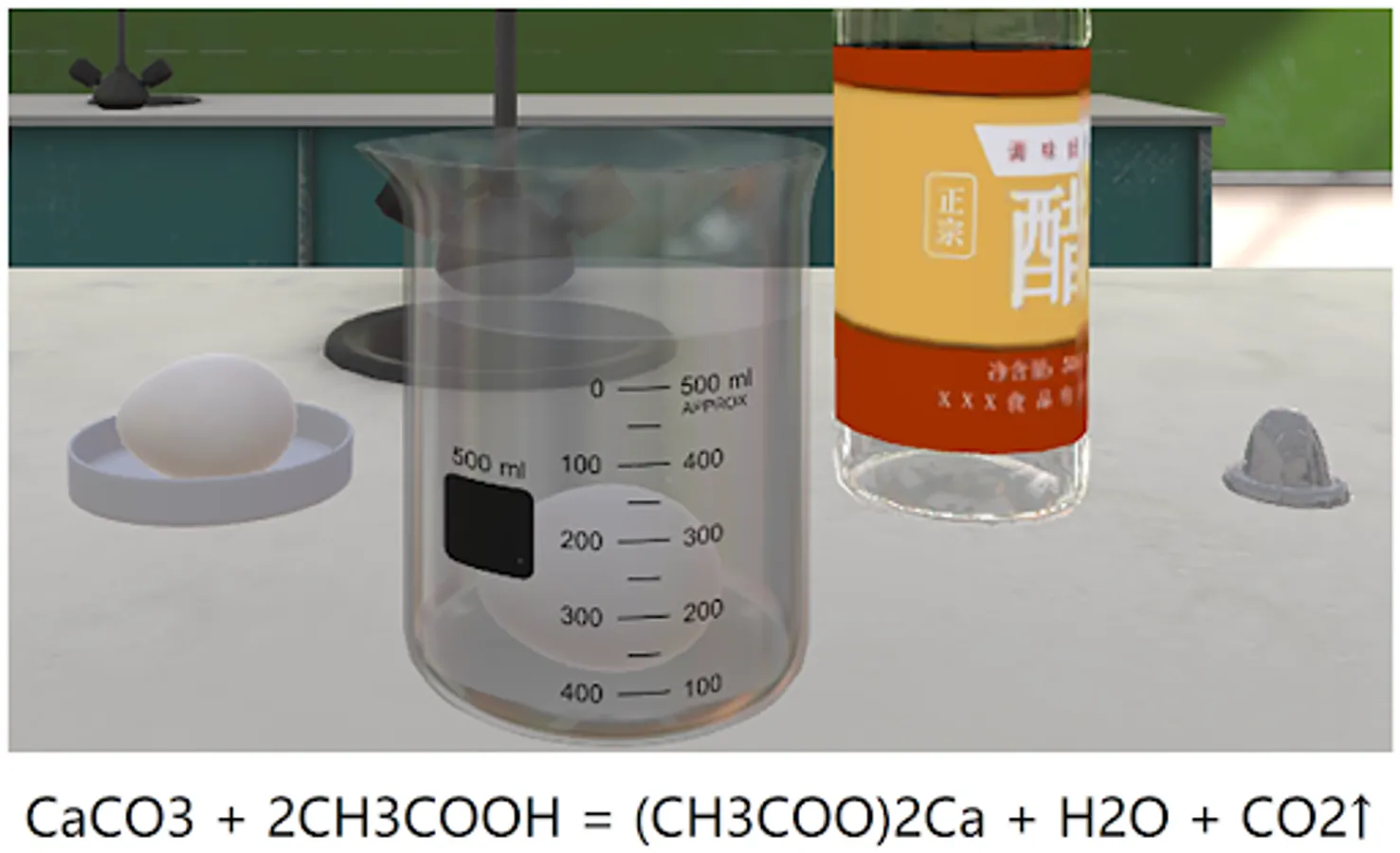
The main component of eggshells is calcium carbonate, and the bubbles from the eggshells soaked in vinegar are carbon dioxide bubbles produced by the dissolution reaction.
Reactant: calcium carbonate in eggshell
Products: carbon dioxide and water
Reaction conditions: contact between eggshell and white vinegar (contact between calcium carbonate and acetic acid)
Chemical formula: CaCO3 + 2CH3COOH = (CH3COO)2Ca + H2O + CO2↑
The main component of eggshells is calcium carbonate, and the bubbles from the eggshells soaked in vinegar are carbon dioxide bubbles produced by the dissolution reaction.
Reactant: calcium carbonate in eggshell
Products: carbon dioxide and water
Reaction conditions: contact between eggshell and white vinegar (contact between calcium carbonate and acetic acid)
Chemical formula: CaCO3 + 2CH3COOH = (CH3COO)2Ca + H2O + CO2↑
Egg With Vinegar Screenshots
Tap to Rate: