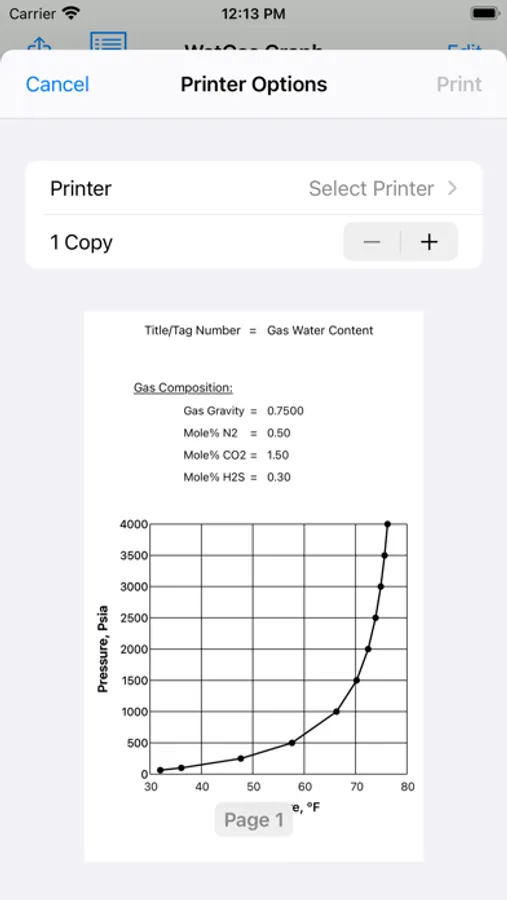
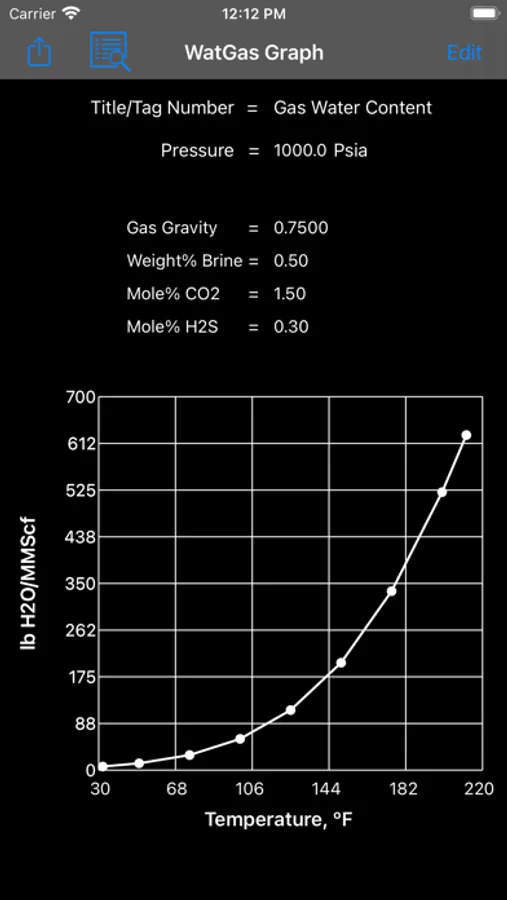
About Gas-Water
Knowing the water content of natural gases is essential to the design and operation of production, dehydration, and transmission systems. Water may condense in production and gathering systems. This may result in hydrate formation and plugging of flow systems and damage to internals of production equipment. Condensed water may form water slugs which will tend to decrease flow efficiency and increase the pressure drop in a line. Presence of free water in pipeline systems may also cause corrosion. If carbon dioxide and/or hydrogen sulphide are present, the gases may form carbonic acid and sulphurous acid respectively if dissolved in water.
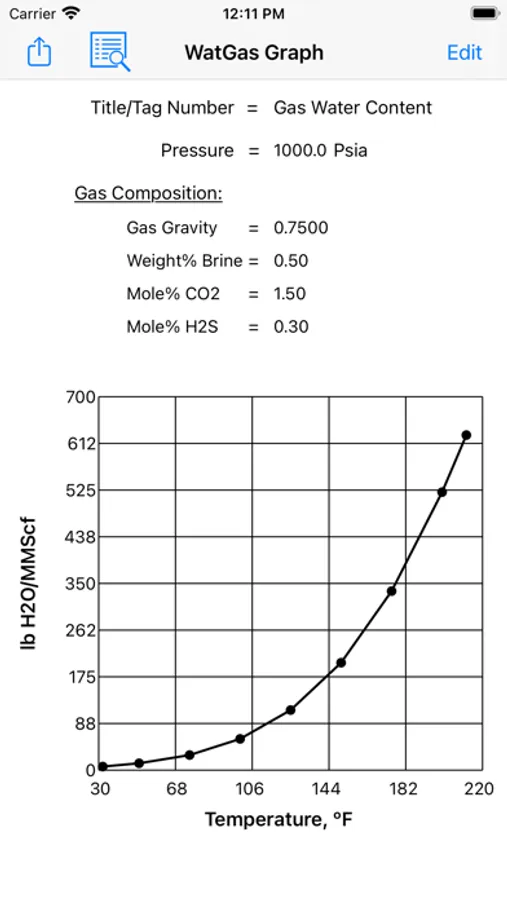
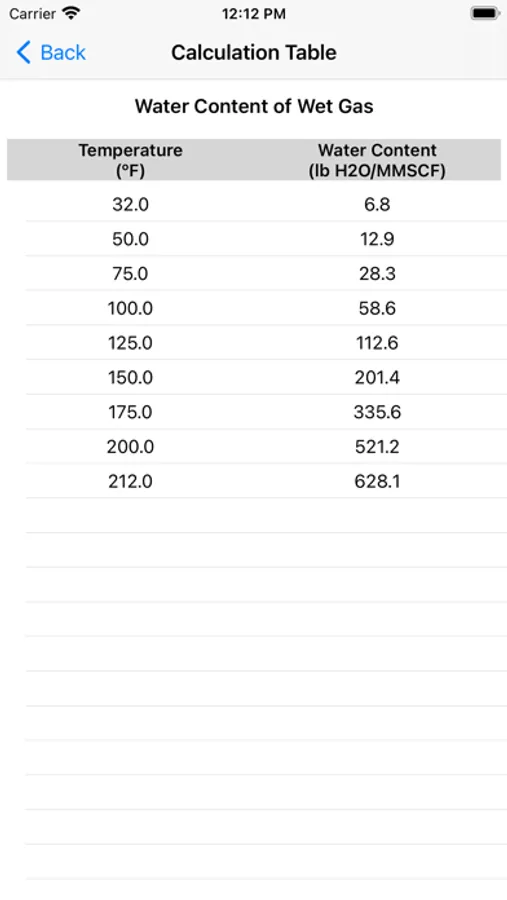
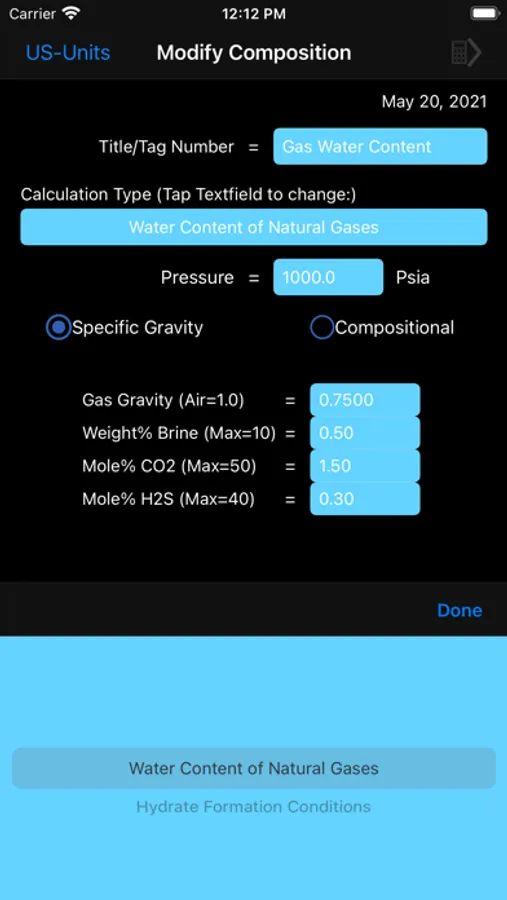
Water content calculations are based on a method developed by Campbell. The method applies the standard physical chemistry equations and the Eykman Molecular Refraction (EMR) combination rules. For water content calculations the approach here uses fugacity coefficient as one of the key factors.
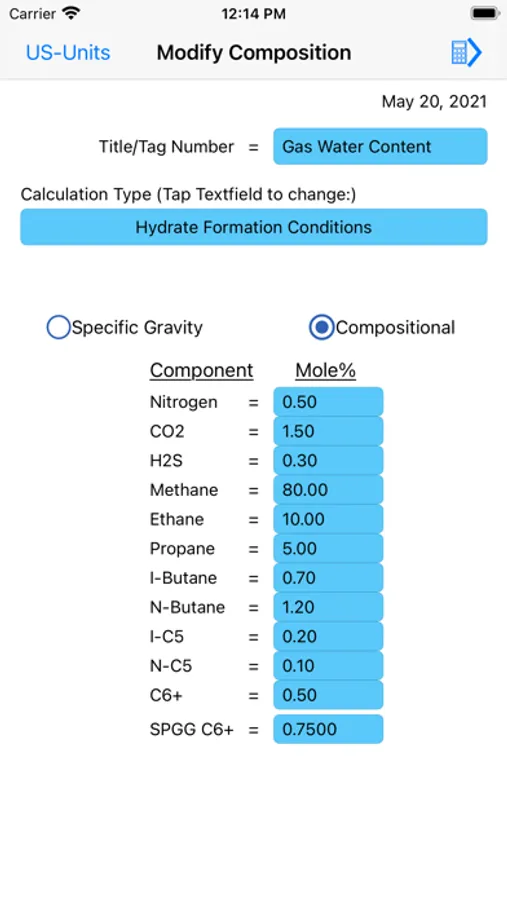
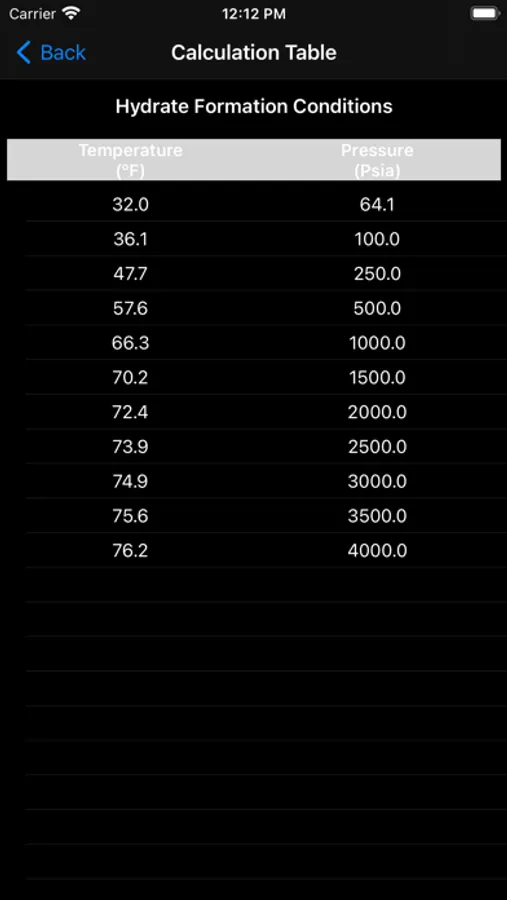
Gas hydrates form when water attaches to molecules of methane, ethane, propane, iso-butane, carbon-dioxide, hydrogen-sulfide, and to a certain extent n-butane. Hydrates form easily with methane, carbon-dioxide, and hydrogen-sulfide. Heavier hydrocarbons tend to inhibit hydrate-formation. It is therefore more difficult to form hydrates of iso-butane and n-butane. Research also shows that benzene, methyl-cyclopentane and cyclohexane may form hydrates in presence of methane or nitrogen.
In this app the hydrate calculations are based on Katz' method for predicting hydrate conditions. Empirically determined vapor-solid equilibrium constants are utilized in predicting hydrate formation conditions.
Water content calculations are based on a method developed by Campbell. The method applies the standard physical chemistry equations and the Eykman Molecular Refraction (EMR) combination rules. For water content calculations the approach here uses fugacity coefficient as one of the key factors.
Gas hydrates form when water attaches to molecules of methane, ethane, propane, iso-butane, carbon-dioxide, hydrogen-sulfide, and to a certain extent n-butane. Hydrates form easily with methane, carbon-dioxide, and hydrogen-sulfide. Heavier hydrocarbons tend to inhibit hydrate-formation. It is therefore more difficult to form hydrates of iso-butane and n-butane. Research also shows that benzene, methyl-cyclopentane and cyclohexane may form hydrates in presence of methane or nitrogen.
In this app the hydrate calculations are based on Katz' method for predicting hydrate conditions. Empirically determined vapor-solid equilibrium constants are utilized in predicting hydrate formation conditions.