About Halmed
Halmed is a free smartphone app for reporting adverse drug reactions of the drug directly to the Agency for Medicinal Products and Medical Devices of Croatia (HALMED). Reporting of adverse drug reactions allow HALMED to collect new information about the previously known adverse reactions and unexpected adverse reactions. Reporting adverse drug reactions directly contributes to safety of drug use. Through this application, it is also possible to track the news published on the HALMED website and receive information on the medicines you choose.
Features of Halmed App:
- The app can be used by healthcare workers and patients
- Report adverse drug reactions directly to HALMED
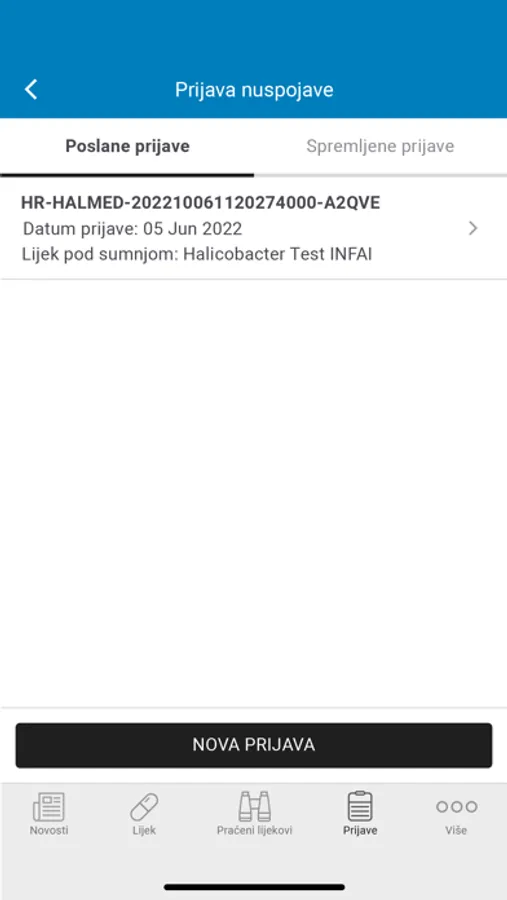
- Review adverse drug reactions you have already submitted
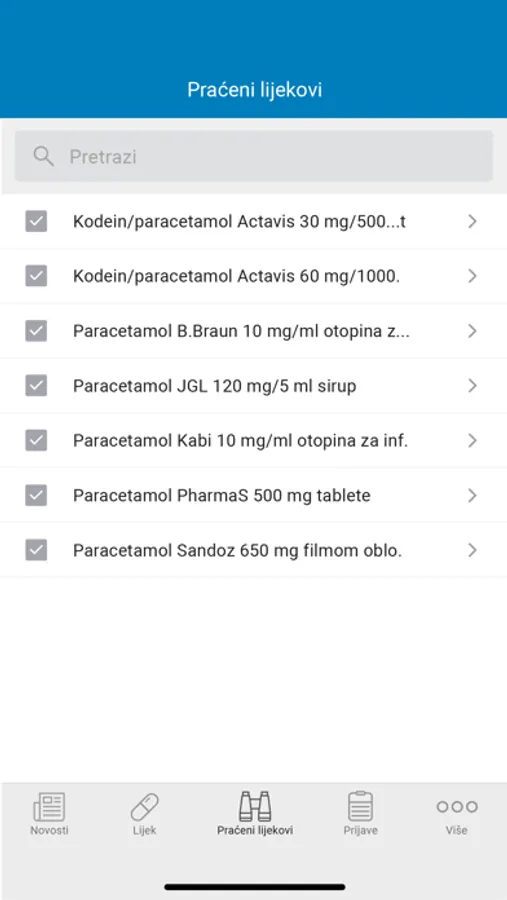
- Keep track of information on selected medicines
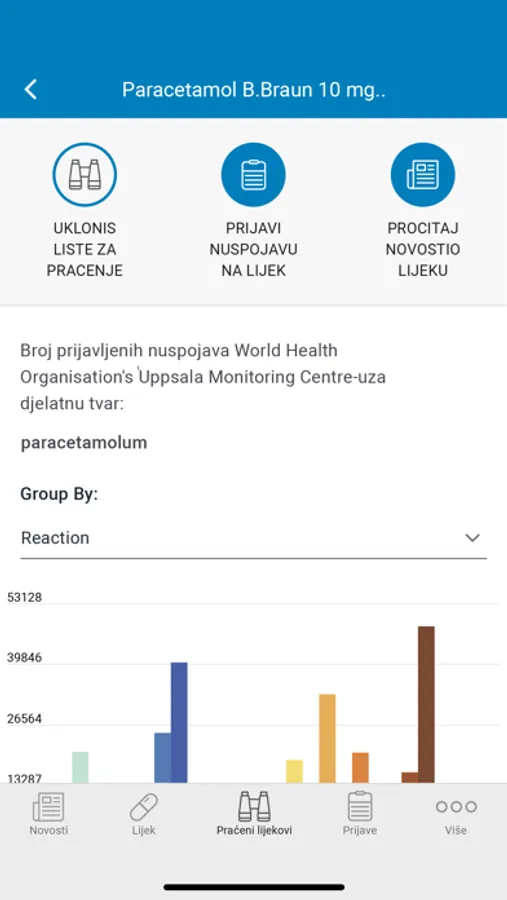
- Check the number of received adverse drug reactions for a particular drug
The Halmed app was developed as part of the WEB-RADR project.
The WEB-RADR project is supported by the EU Public-Private Partnership Innovative Medicines Initiative
Joint Undertaking based on the Grant Agreement no. 115632, whose resources are made up of financial contributions from the 7th European Union Framework Program (FP7 / 2007-2013) and contributes in kind to the companies of the European Federation of Pharmaceutical Industries and Associations (EFPIA).
More information on the IMI initiative is available under the link www.imi.europa.eu
Features of Halmed App:
- The app can be used by healthcare workers and patients
- Report adverse drug reactions directly to HALMED
- Review adverse drug reactions you have already submitted
- Keep track of information on selected medicines
- Check the number of received adverse drug reactions for a particular drug
The Halmed app was developed as part of the WEB-RADR project.
The WEB-RADR project is supported by the EU Public-Private Partnership Innovative Medicines Initiative
Joint Undertaking based on the Grant Agreement no. 115632, whose resources are made up of financial contributions from the 7th European Union Framework Program (FP7 / 2007-2013) and contributes in kind to the companies of the European Federation of Pharmaceutical Industries and Associations (EFPIA).
More information on the IMI initiative is available under the link www.imi.europa.eu