About IEGBBR Compendium
The International Experts Groups of Biosafety and Biosecurity Regulators (IEGBBR) Mobile Application of Biosafety, Biosecurity and Dual-Use Oversight provides detailed descriptions of the national oversight systems among the 11 IEGBBR member countries (Australia, Canada, Denmark, France, Germany, Japan, Netherlands, Singapore, Switzerland, United Kingdom, United States). It allows users to search and contrast desired aspects of the biosafety, biosecurity and dual-use oversight for any or all of the IEGBBR member countries.
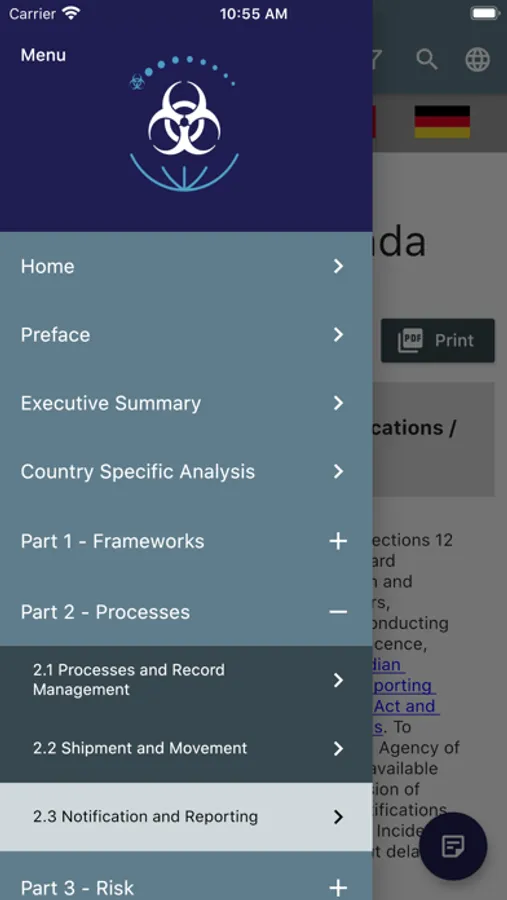
The mobile app has two modules:
1. The Compendium of International Biosafety and Biosecurity Oversight Systems for Human and Animal Pathogens and Toxins
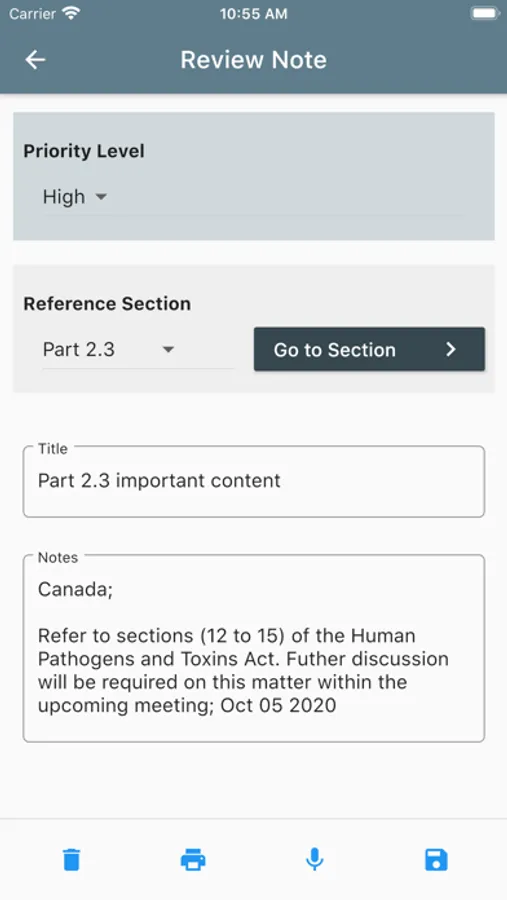
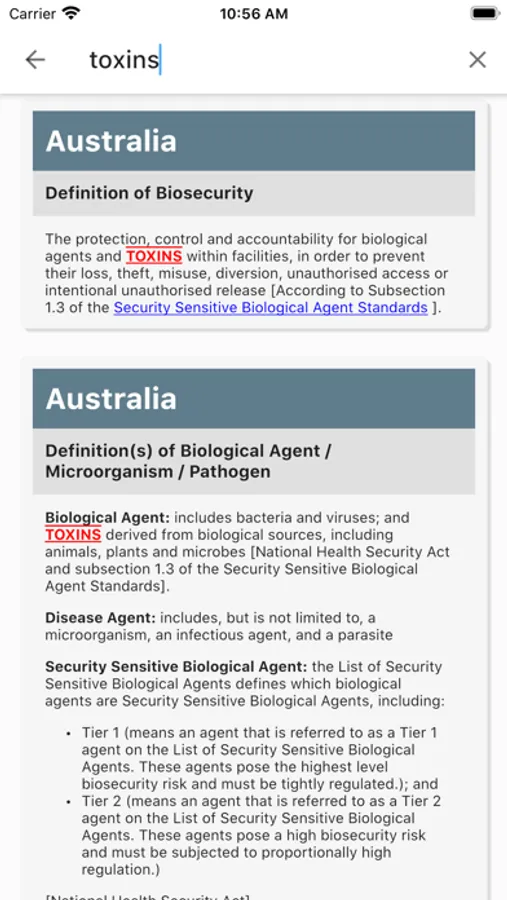
Content includes statutes and regulations and their regulatory authorities; biosafety- and biosecurity-related definitions and biosafety risk group designations; purpose, scope and activities; regulated pathogens and toxins; standards and policy instruments; processes and record management requirements for regulatory authorization; shipment and movement of biological agents and toxins; notification and reporting; risk assessment requirements; compliance promotion, monitoring, verification, and enforcement; inspection schemes; accountability and personnel security requirements; non-security personnel requirements and training; and biosafety and biosecurity outreach and awareness initiatives.
2. The Review of Oversight of Dual-Use (DU) in Life Sciences
Content includes governance approaches and definitions; export controls and international agreements; statutes and regulations to address export controls; export control program requirements; tools and resources; national biosafety and biosecurity oversight measures; statutes and regulations of primary relevance to DU; oversight measures of the regulatory oversight system; other oversight approaches (institutional, funding allocation and publication); transportation and transfer of DU goods and information; risk-benefit assessment and risk mitigation; processes related to ethical and societal implications; compliance monitoring and enforcement; training, outreach and raising awareness; and challenges with DU governance.
The mobile app will serve as a reference tool for countries that aim to develop or strengthen their national biosafety, biosecurity or dual-use oversight capacities. By providing 11 examples of national oversight systems, it can remove the necessity for extensive legwork prior to the development and implementation of oversight. The IEGBBR mobile app can be used hand in hand with other complementary capacity building reference tools that are available, such as the Joint External Evaluation assessments under the International Health Regulations and Canada’s Analytical Approach. The Compendium contributes to ongoing international biosafety and biosecurity efforts that exist internationally, including the Global Health Security Agenda, and strengthens national/regional compliance with commitments under the World Health Organization International Health Regulations, United Nations Security Council Resolution 1540, and the Biological and Toxin Weapons Convention.
The mobile app has two modules:
1. The Compendium of International Biosafety and Biosecurity Oversight Systems for Human and Animal Pathogens and Toxins
Content includes statutes and regulations and their regulatory authorities; biosafety- and biosecurity-related definitions and biosafety risk group designations; purpose, scope and activities; regulated pathogens and toxins; standards and policy instruments; processes and record management requirements for regulatory authorization; shipment and movement of biological agents and toxins; notification and reporting; risk assessment requirements; compliance promotion, monitoring, verification, and enforcement; inspection schemes; accountability and personnel security requirements; non-security personnel requirements and training; and biosafety and biosecurity outreach and awareness initiatives.
2. The Review of Oversight of Dual-Use (DU) in Life Sciences
Content includes governance approaches and definitions; export controls and international agreements; statutes and regulations to address export controls; export control program requirements; tools and resources; national biosafety and biosecurity oversight measures; statutes and regulations of primary relevance to DU; oversight measures of the regulatory oversight system; other oversight approaches (institutional, funding allocation and publication); transportation and transfer of DU goods and information; risk-benefit assessment and risk mitigation; processes related to ethical and societal implications; compliance monitoring and enforcement; training, outreach and raising awareness; and challenges with DU governance.
The mobile app will serve as a reference tool for countries that aim to develop or strengthen their national biosafety, biosecurity or dual-use oversight capacities. By providing 11 examples of national oversight systems, it can remove the necessity for extensive legwork prior to the development and implementation of oversight. The IEGBBR mobile app can be used hand in hand with other complementary capacity building reference tools that are available, such as the Joint External Evaluation assessments under the International Health Regulations and Canada’s Analytical Approach. The Compendium contributes to ongoing international biosafety and biosecurity efforts that exist internationally, including the Global Health Security Agenda, and strengthens national/regional compliance with commitments under the World Health Organization International Health Regulations, United Nations Security Council Resolution 1540, and the Biological and Toxin Weapons Convention.