About UDILink
UDILink provides medical professionals with easy, instant and accurate communication channels for medical device complaints and feedback to manufacturers as recommended by the FDA and similar authorities. Professionals can use their smartphone to:
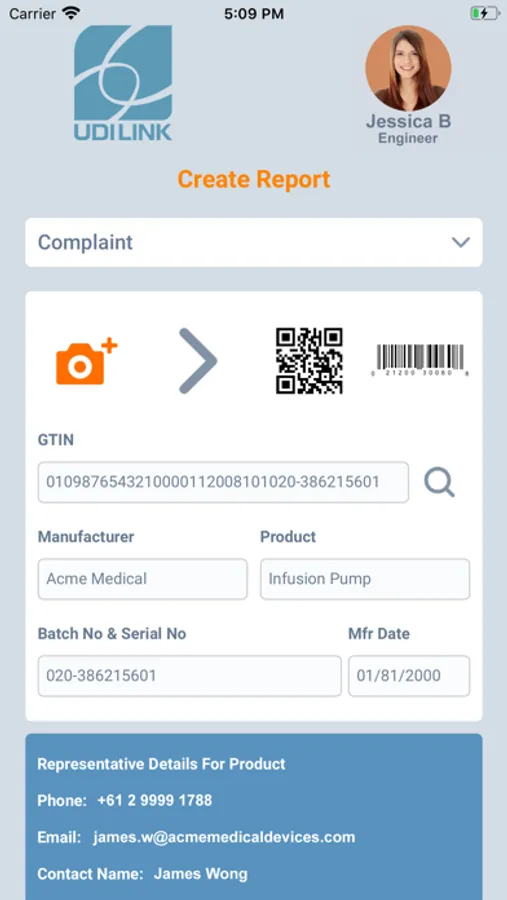
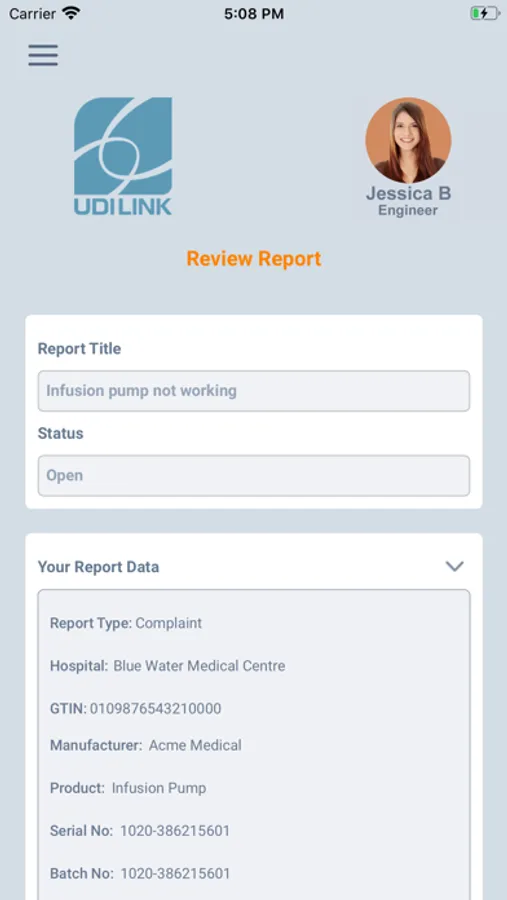
1. Scan the Unique Device Identifier (UDI) barcode, instantly identifying the manufacturer, device model and serial / batch data.
2. Register themselves and/or the hospital as verified users, saving repeated self-identification.
3. Set communication preferences and urgencies – from detailed tracking to just outcome notification.
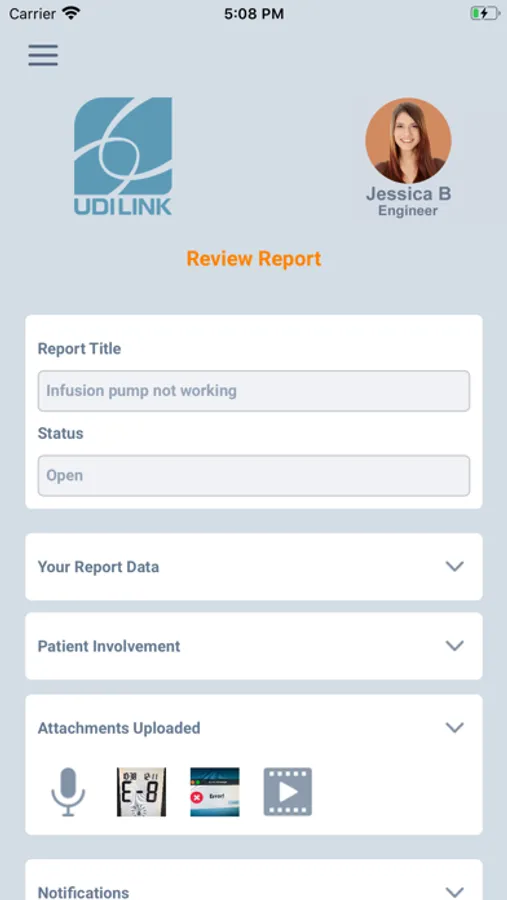
4. Provide easy to fill the description of the problem.
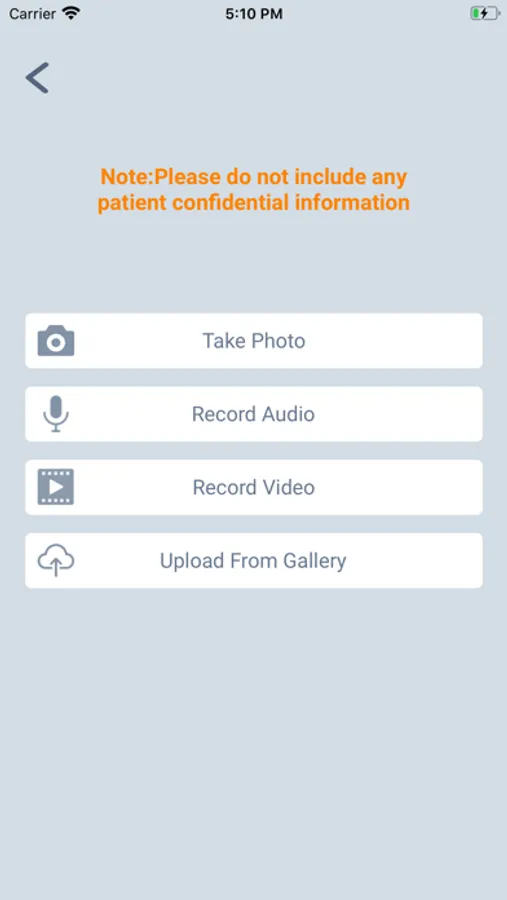
5. Upload photo and video evidence straight from your smartphone.
With UDILInk, you can create and edit a full report on the spot using your smartphone, and send it to your usual company representative or the manufacturer specified call centre.
UDILink is intended for hospital clinical and technical staff as well as consumers of medical devices who may not have easy access to the manufacturer.
UDILink complies with security and privacy laws and helps companies fulfil compliance with post-market surveillance and reporting.
Benefits for users:
• Quick, efficient, accurate complaint and feedback reporting platform for users – hospital nurses, unit managers, technicians, service staff, consumers.
• Make report at the time of the problem, when memory fresh, witnesses are present, and data is available, not days later.
• Identify full device details with one snapshot using the mandated UDI barcode on every device.
• Capture evidence of malfunction which may not be easily reproducible later.
• Easily capture and upload photo and video evidence at the time of the problem – product appearance, software behaviour or error messages.
• Guided complaint description – ensures key descriptive, risk and urgency information avoiding multiple inquiries for details, phone calls, representative visits.
• Instant lookup of manufacturer contacts
• Instant updates on complaint progress within the App.
• Update complaint data any time
• Multimedia text with a company representative
Benefits to Manufacturers
• Obtain more and higher quality postmarket feedback, improving product safety, performance.
• Improve ISO13485 / 21CFR820 post-market feedback compliance given the increasing regulatory focus on this area; including platform in PMS Plan and data in PMCF / PSUR.
• Obtain timely feedback that is well described, complete, accurate and supported by actionable evidence.
• Reduce call centre and support staff load from chasing customers with phone calls for details and follow up.
• Reduce multiple Field Engineer callouts to identify the device, get the full story from witnesses and then still often fail to define or reproduce the problem.
• Improve reliability of postmarket trend analysis, reduce uncertainty and false investigations.
• Use complaint handling as promotion; Improve customer satisfaction and loyalty.
Benefits to Patients:
• Safer, more effective medical devices.
1. Scan the Unique Device Identifier (UDI) barcode, instantly identifying the manufacturer, device model and serial / batch data.
2. Register themselves and/or the hospital as verified users, saving repeated self-identification.
3. Set communication preferences and urgencies – from detailed tracking to just outcome notification.
4. Provide easy to fill the description of the problem.
5. Upload photo and video evidence straight from your smartphone.
With UDILInk, you can create and edit a full report on the spot using your smartphone, and send it to your usual company representative or the manufacturer specified call centre.
UDILink is intended for hospital clinical and technical staff as well as consumers of medical devices who may not have easy access to the manufacturer.
UDILink complies with security and privacy laws and helps companies fulfil compliance with post-market surveillance and reporting.
Benefits for users:
• Quick, efficient, accurate complaint and feedback reporting platform for users – hospital nurses, unit managers, technicians, service staff, consumers.
• Make report at the time of the problem, when memory fresh, witnesses are present, and data is available, not days later.
• Identify full device details with one snapshot using the mandated UDI barcode on every device.
• Capture evidence of malfunction which may not be easily reproducible later.
• Easily capture and upload photo and video evidence at the time of the problem – product appearance, software behaviour or error messages.
• Guided complaint description – ensures key descriptive, risk and urgency information avoiding multiple inquiries for details, phone calls, representative visits.
• Instant lookup of manufacturer contacts
• Instant updates on complaint progress within the App.
• Update complaint data any time
• Multimedia text with a company representative
Benefits to Manufacturers
• Obtain more and higher quality postmarket feedback, improving product safety, performance.
• Improve ISO13485 / 21CFR820 post-market feedback compliance given the increasing regulatory focus on this area; including platform in PMS Plan and data in PMCF / PSUR.
• Obtain timely feedback that is well described, complete, accurate and supported by actionable evidence.
• Reduce call centre and support staff load from chasing customers with phone calls for details and follow up.
• Reduce multiple Field Engineer callouts to identify the device, get the full story from witnesses and then still often fail to define or reproduce the problem.
• Improve reliability of postmarket trend analysis, reduce uncertainty and false investigations.
• Use complaint handling as promotion; Improve customer satisfaction and loyalty.
Benefits to Patients:
• Safer, more effective medical devices.