NBIR Device Tracking
American Society of Plastic Surgeons
3.4 ★
10 ratings
Free
AppRecs review analysis
AppRecs rating 3.7. Trustworthiness 65 out of 100. Review manipulation risk 22 out of 100. Based on a review sample analyzed.
★★★☆☆
3.7
AppRecs Rating
Ratings breakdown
5 star
60%
4 star
0%
3 star
0%
2 star
0%
1 star
40%
What to know
✓
Low review manipulation risk
22% review manipulation risk
About NBIR Device Tracking
The NBIR Device Tracking App is a HIPAA-compliant app that can be used to quickly capture data that is required for the purposes of device tracking – a federally-mandated requirement of manufacturers of breast implants. This means physicians can simultaneously fulfill their device tracking requirements for Allergan, Mentor and Sientra by participating in the NBIR.
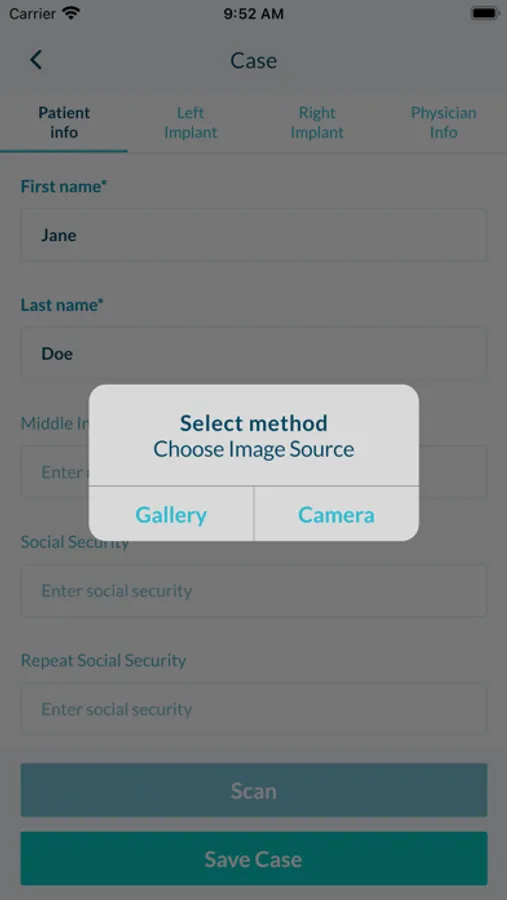
The app uses OCR technology to scan and capture data from patient demographic forms and breast implant device barcodes. Captured data is securely packaged in a case report form and submitted to NBIR.
The NBIR is a collaboration between The Plastic Surgery Foundation, the FDA, and breast implant device manufacturers for the purpose of strengthening the post-market surveillance infrastructure for current and future breast implant devices. For more information, please visit thepsf.org/NBIR
The app uses OCR technology to scan and capture data from patient demographic forms and breast implant device barcodes. Captured data is securely packaged in a case report form and submitted to NBIR.
The NBIR is a collaboration between The Plastic Surgery Foundation, the FDA, and breast implant device manufacturers for the purpose of strengthening the post-market surveillance infrastructure for current and future breast implant devices. For more information, please visit thepsf.org/NBIR