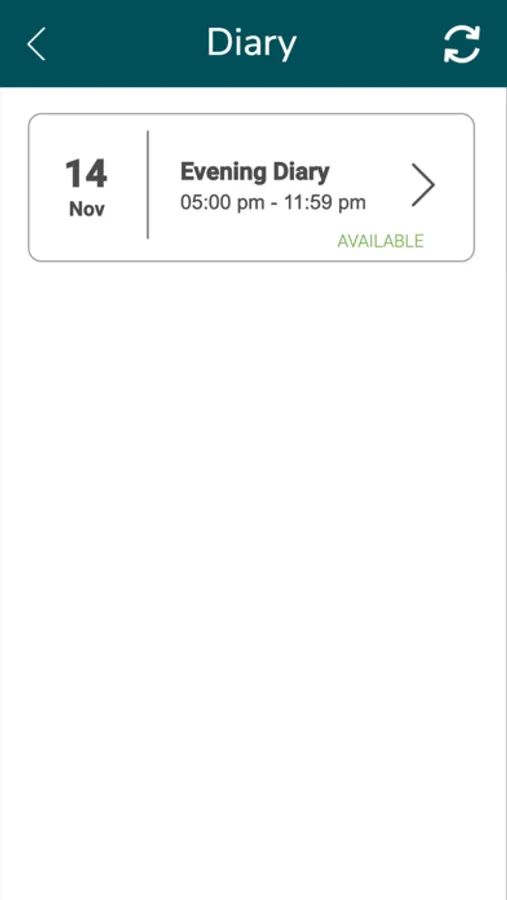
About FSD201-03
Application in support of drug in clinical trials to determine safety for use in humans. eDiary collects signs and symptoms of reactions for 14 days after the drug administration and reports data to study investigators. Study participants will receive notification reminders to complete their daily eDiary.
Access to participate in this study requires informed consent by medical personnel. Only after consenting will participants receive an invitation to complete eDiaries.
snapIoT and its platforms are HIPAA, California Privacy Rights Act, and GDPR compliant
Access to participate in this study requires informed consent by medical personnel. Only after consenting will participants receive an invitation to complete eDiaries.
snapIoT and its platforms are HIPAA, California Privacy Rights Act, and GDPR compliant