SITC Immunotherapy Guidelines
SOCIETY FOR IMMUNOTHERAPY OF CANCER, INC.
4.8 ★
79 ratings
Free
In this medical reference app, you can access guidelines and interactive tools related to cancer immunotherapy. Includes search functionality, bookmarking, and educational resources.
AppRecs review analysis
AppRecs rating 4.4. Trustworthiness 0 out of 100. Review manipulation risk 0 out of 100. Based on a review sample analyzed.
★★★★☆
4.4
AppRecs Rating
Ratings breakdown
5 star
91%
4 star
5%
3 star
0%
2 star
1%
1 star
3%
What to know
✓
High user satisfaction
91% of sampled ratings are 5 stars
✓
Authentic reviews
No red flags detected
About SITC Immunotherapy Guidelines
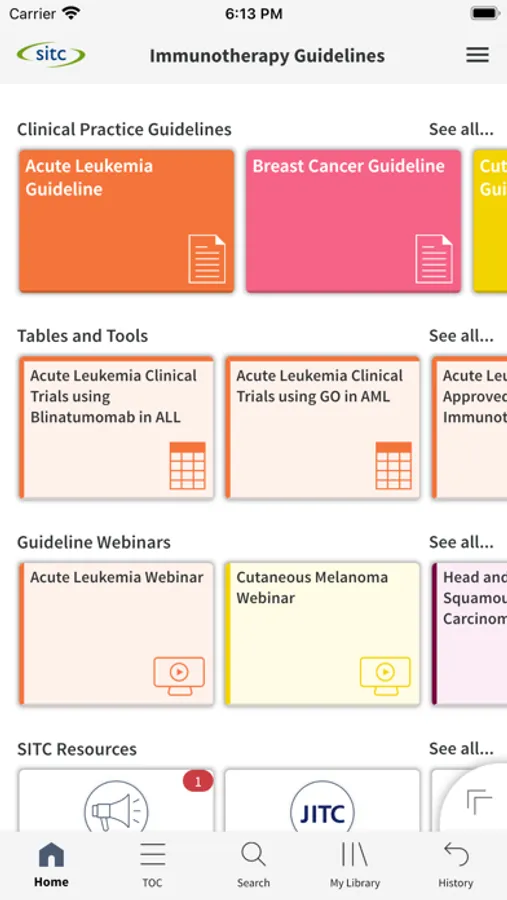
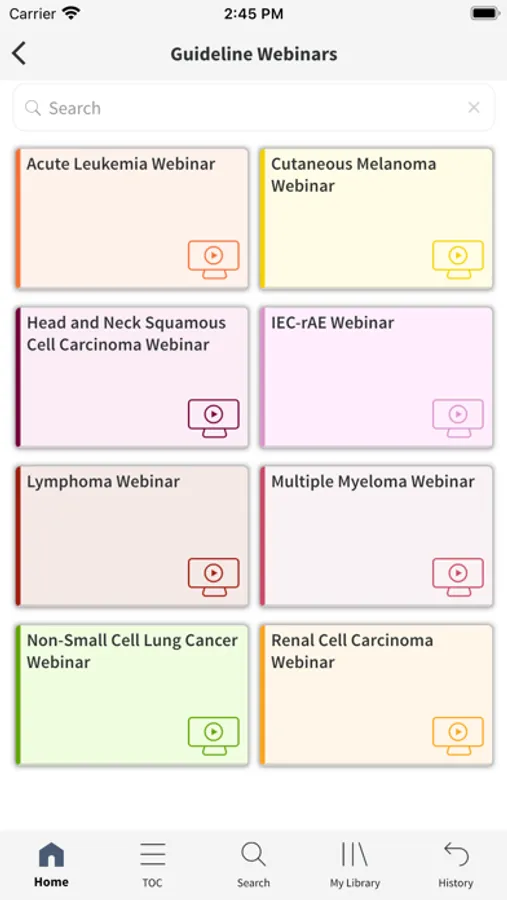
The Society for Immunotherapy of Cancer (SITC) Clinical Practice Guidelines (CPG) Mobile App is the first and only tool of its kind, offering direct, easy, portable access to SITC’s CPGs via phone or tablet. Highlighting key information from SITC’s published guidelines, the SITC CPG App features evidence- and expert consensus based-recommendations on all aspects of immunotherapy treatment as well as interactive tools and companion educational resources. From biomarkers and therapy selection to quality of life and management of immune-related adverse events, busy clinicians will find the SITC CPG App as the go-to resource on when and how to use immunotherapy to help improve outcomes for patients with cancer.
Key App Features
*Clean and simple navigation with a modern interface
*Interactive tools and tables at your fingertips
*Advanced search functionality for fast access to the content clinicians need
*Bookmarking and annotation capabilities throughout for future fast-reference
*Timely updates when new practice-changing data or approvals become available
*Companion educational offerings to enhance understanding of guideline recommendations
*Free download with open-access content
About SITC’s Guidelines
SITC’s Clinical Practice Guidelines (CPGs) offer a vital resource for the practicing oncology community by providing practical, actionable recommendations to help cancer care providers develop immunotherapy treatment plans for their patients. The CPGs are developed by multi-disciplinary panels of experts who draw from their own practical experience as well as evidence in the published literature and clinical trial data to develop evidence- and consensus-based recommendations on when and how to use immunotherapy to help improve outcomes for patients with cancer. SITC uses as a model the Institute of Medicine's 2011 "Standards for Developing Trustworthy Clinical Practice Guidelines" to ensure a fair, transparent and balanced process for creating the CPGs. Each CPG expert panel is comprised of a comprehensive mix of academic physicians and researchers, nurses, patients, and patient advocates invited from institutions across the United States. The guidelines are reviewed on an ongoing basis to account for newly available clinical trial data and FDA approvals.
About SITC
SITC is a 501(c)(3) not-for-profit medical professional society of influential research scientists, physician scientists, clinicians, patients, patient advocates, government representatives and industry leaders dedicated to improving cancer patient outcomes by advancing both the science behind – and the clinical application of – cancer immunotherapy. SITC aims to one day make the word “cure” a reality for cancer patients everywhere. www.sitcancer.org
sitcancer.org/CPG-app
On social - #SITCGuidelines
Key App Features
*Clean and simple navigation with a modern interface
*Interactive tools and tables at your fingertips
*Advanced search functionality for fast access to the content clinicians need
*Bookmarking and annotation capabilities throughout for future fast-reference
*Timely updates when new practice-changing data or approvals become available
*Companion educational offerings to enhance understanding of guideline recommendations
*Free download with open-access content
About SITC’s Guidelines
SITC’s Clinical Practice Guidelines (CPGs) offer a vital resource for the practicing oncology community by providing practical, actionable recommendations to help cancer care providers develop immunotherapy treatment plans for their patients. The CPGs are developed by multi-disciplinary panels of experts who draw from their own practical experience as well as evidence in the published literature and clinical trial data to develop evidence- and consensus-based recommendations on when and how to use immunotherapy to help improve outcomes for patients with cancer. SITC uses as a model the Institute of Medicine's 2011 "Standards for Developing Trustworthy Clinical Practice Guidelines" to ensure a fair, transparent and balanced process for creating the CPGs. Each CPG expert panel is comprised of a comprehensive mix of academic physicians and researchers, nurses, patients, and patient advocates invited from institutions across the United States. The guidelines are reviewed on an ongoing basis to account for newly available clinical trial data and FDA approvals.
About SITC
SITC is a 501(c)(3) not-for-profit medical professional society of influential research scientists, physician scientists, clinicians, patients, patient advocates, government representatives and industry leaders dedicated to improving cancer patient outcomes by advancing both the science behind – and the clinical application of – cancer immunotherapy. SITC aims to one day make the word “cure” a reality for cancer patients everywhere. www.sitcancer.org
sitcancer.org/CPG-app
On social - #SITCGuidelines