About Genesis Study
A 12-month randomised, double-blind, controlled, international multicentre trial comparing changes in cigarette consumption after switching to high or low nicotine strength e-cigarettes in smokers with Schizophrenia spectrum disorders. This will be a multicenter, 12-months prospective trial, utilizing a randomized, double-blind, 2-arm parallel, switching design to compare effectiveness, tolerability, acceptability, and pattern of use between high (JUUL 5% nicotine) and low nicotine strength devices (JUUL 1.5% nicotine) in adult smokers with schizophrenia spectrum disorders. The study will take place at 5 sites: 1 in the UK (London) and possibly 4 in Italy.
DISCLAIMER : To seek a doctor’s advice in addition to using this app and before making any medical decisions.
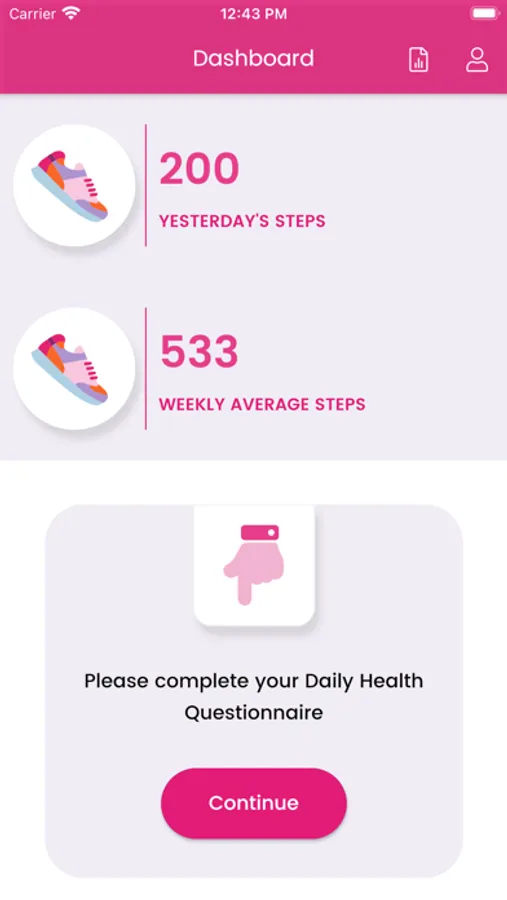
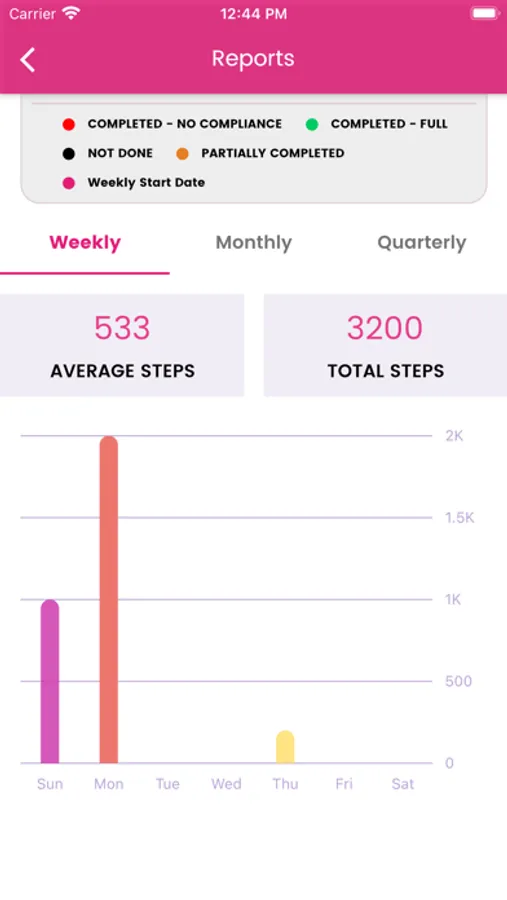
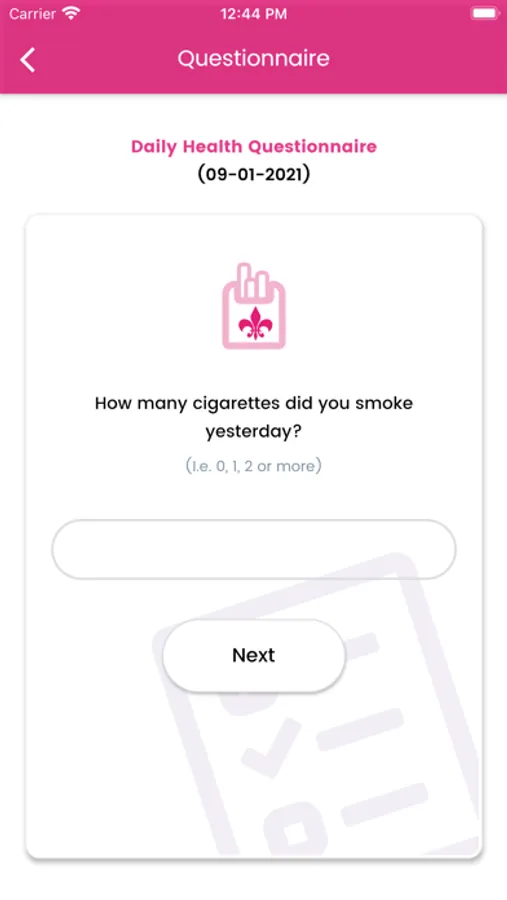
We are using HealthKit to get step count to display on Health Dairy screen and graph on Report screen. We also send daily step count to server with questionary for study purpose.
DISCLAIMER : To seek a doctor’s advice in addition to using this app and before making any medical decisions.
We are using HealthKit to get step count to display on Health Dairy screen and graph on Report screen. We also send daily step count to server with questionary for study purpose.