About Bobbie Study
Welcome to the Bobbie Study:
The Bobbie app is a proprietary platform of ObvioHealth being used to manage a trial to study use of an investigational infant formula and how it affect infant growth and tolerance in comparison to current market formula or breast milk.
The Bobbie app removes the costly overhead of physical site visits and brings the trial directly to the mobile device of each subject.
The result? Robust data collection, increased compliance, faster time to completion, and average cost savings of ~50% vs. traditional on-site trials.
Subject Journey
A subject who is identified as eligible for this study during pre-screening will be invited to download the Bobbie App via e-mail.
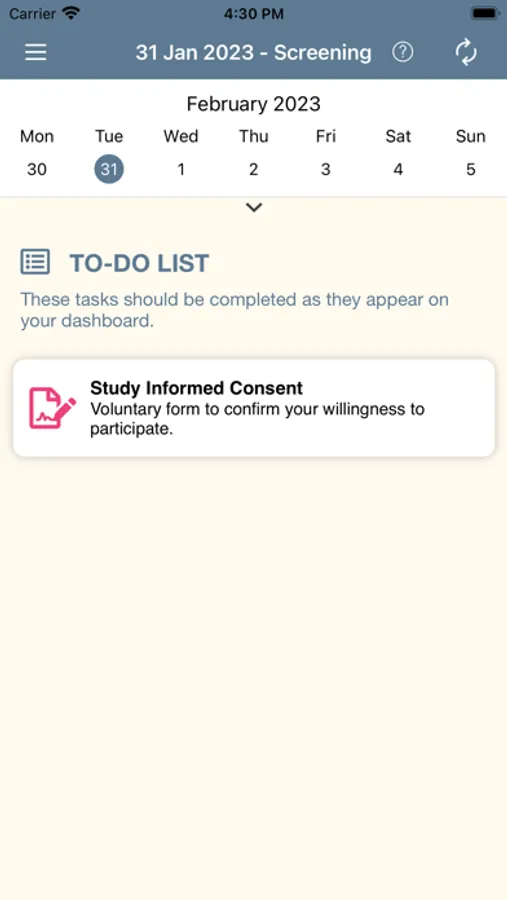
Upon download, the participants create a Bobbie account and complete the Informed Consent process. A series of screens explains the parameters of the study, including:
o Privacy Policy
o Data Gathering & Usage
o Study Tasks & Surveys
o Time Commitment
o Option to Withdraw
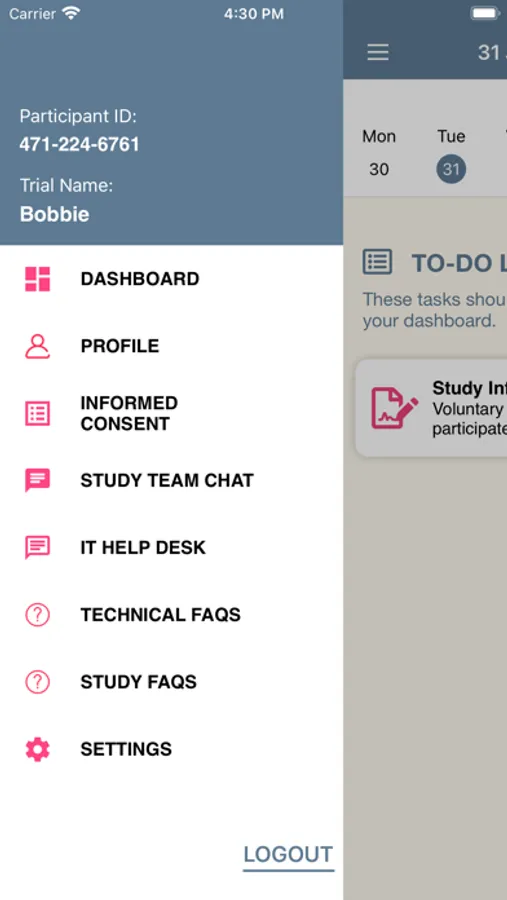
Subjects will have the opportunity to connect with a member of the study team to ask questions prior to signing the informed consent form.
Intervention Period: Daily Tasks
Subjects will contribute data to the study by completing questionnaires and data uploads over a 7-day baseline period and 16-week active trial period.
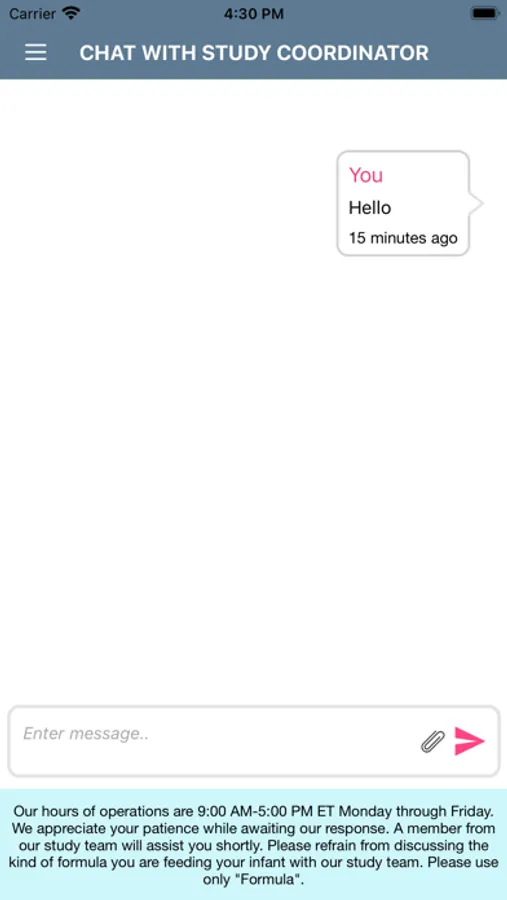
Subjects can interact with the study team via secure chat as needed.
The Bobbie app is a proprietary platform of ObvioHealth being used to manage a trial to study use of an investigational infant formula and how it affect infant growth and tolerance in comparison to current market formula or breast milk.
The Bobbie app removes the costly overhead of physical site visits and brings the trial directly to the mobile device of each subject.
The result? Robust data collection, increased compliance, faster time to completion, and average cost savings of ~50% vs. traditional on-site trials.
Subject Journey
A subject who is identified as eligible for this study during pre-screening will be invited to download the Bobbie App via e-mail.
Upon download, the participants create a Bobbie account and complete the Informed Consent process. A series of screens explains the parameters of the study, including:
o Privacy Policy
o Data Gathering & Usage
o Study Tasks & Surveys
o Time Commitment
o Option to Withdraw
Subjects will have the opportunity to connect with a member of the study team to ask questions prior to signing the informed consent form.
Intervention Period: Daily Tasks
Subjects will contribute data to the study by completing questionnaires and data uploads over a 7-day baseline period and 16-week active trial period.
Subjects can interact with the study team via secure chat as needed.